① Fosun Pharmaceutical's revenue for innovative drugs in the first half of the year exceeded 3.7 billion yuan, accounting for about 18% of overall revenue. Wu Yifang revealed that the innovative drug business will aim to reach a compound annual growth rate of 30% in the next few years. ② Wu Yifang said that the privatization of Fuhong Hanlin is in progress, and through privatization, Fuhong Hanlin's entire business and pipeline will be deeply integrated with Fosun Pharmaceuticals.
“Science and Technology Innovation Board Daily”, August 29 (Reporter Zheng Bingxun) “At the end of last year, the pressure brought about by COVID-19 was clear. In the first half of this year, we began to enter a phase of restorative growth.” At the performance exchange meeting held on the 28th, Wu Yifang, chairman of Fosun Pharmaceutical (600196.SH), said.
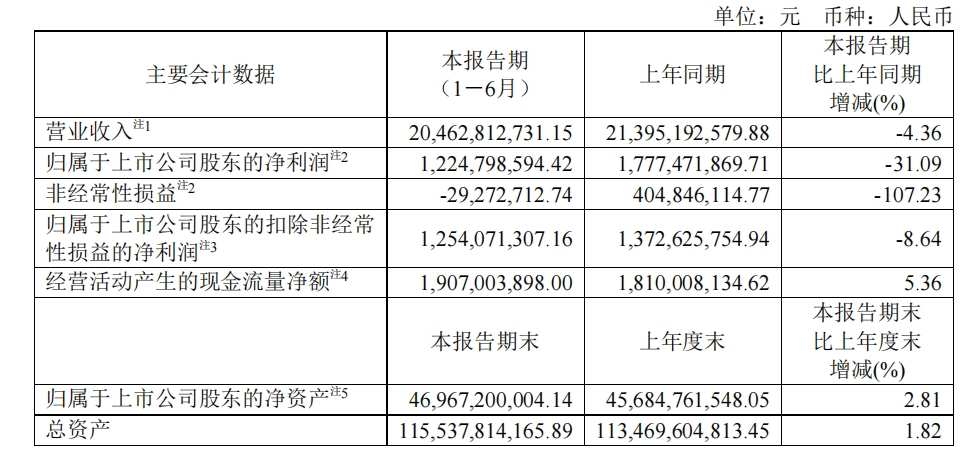
According to the 2024 semi-annual report, Fosun Pharmaceutical achieved revenue of 20.463 billion yuan, a year-on-year decrease of 4.36 percentage points, and net profit to mother of 1.225 billion yuan, a year-on-year decrease of 31.09%. Fosun Pharmaceutical said that without the impact of the year-on-year decline in COVID-related products, revenue for the first half of the year increased 5.31% year on year.
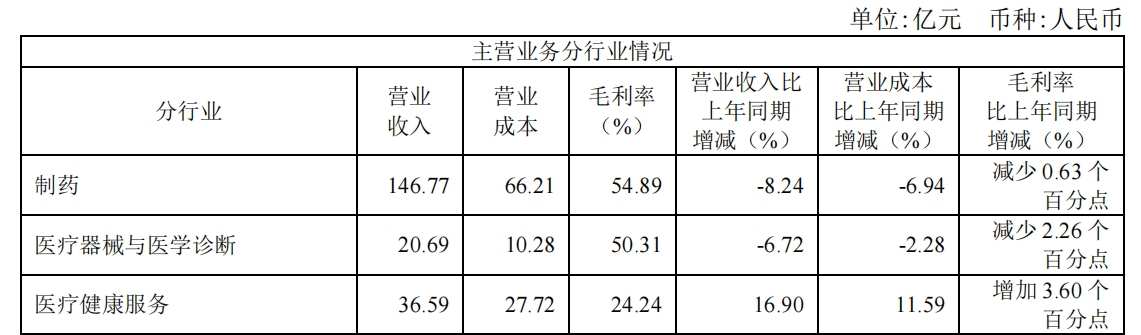
 Currently, according to different business segments, Fosun Pharmaceutical's revenue mainly comes from pharmaceuticals, medical devices, medical diagnosis, and healthcare services. The pharmaceutical sector is still Fosun Pharmaceutical's largest revenue source. The sector achieved revenue of 14.677 billion yuan during the reporting period, a year-on-year decrease of 8.24%, accounting for 71.72% of revenue.
Currently, according to different business segments, Fosun Pharmaceutical's revenue mainly comes from pharmaceuticals, medical devices, medical diagnosis, and healthcare services. The pharmaceutical sector is still Fosun Pharmaceutical's largest revenue source. The sector achieved revenue of 14.677 billion yuan during the reporting period, a year-on-year decrease of 8.24%, accounting for 71.72% of revenue.
In response to the decline in revenue from the pharmaceutical sector, Wu Yifang explained at the performance conference, “On the one hand, collection and medical insurance price negotiations have had a certain impact on the revenue of the pharmaceutical sector. On the other hand, innovative drugs that participated in health insurance negotiations at the end of last year are still in the process of entering hospitals, and sales in the first and second quarters are still low.” Wu Yifang said that the growth of innovative drugs will increase in the third quarter. “The overall revenue growth of innovative drugs will aim to reach a compound annual growth rate of 30% in the next few years.”
In the first half of the year, a total of 9 indications for the 4 innovative drugs/biosimilar drugs developed and approved by Fosun Pharmaceutical were approved at home and abroad, and 38 generic drugs were approved at home and abroad. Among them, the revenue of innovative drugs exceeded 3.7 billion yuan, accounting for about 18% of the overall revenue and about 25% of the pharmaceutical sector.
“In the future, conventional generic drugs will probably maintain single-digit growth. Innovative drugs are estimated to account for half of revenue over a 5-year cycle, or even higher.” Wu Yifang continued, “But we will still stick to the combination of imitation and innovation. Profits from generic drugs largely support our investment in innovative drugs.”
Fuhong Hanlin (2696.HK), a subsidiary of Fosun Pharmaceutical Holdings, has made a significant contribution in terms of innovative drug revenue.
Fuhong Hanlin achieved revenue of 2.746 billion yuan in the first half of the year, up about 9.8% year on year, and net profit of 0.386 billion yuan, up about 61.0% year on year. The total sales revenue of the five products was 2.479 billion yuan, an increase of 15.2% over the previous year. Among them, injectable trastuzumab (Han Quyou), slulizumab (Hans form), and bevacizumab (Hanbetai) achieved sales revenue of 1.474 billion yuan, 0.678 billion yuan, and 0.087 billion yuan respectively.
It is worth mentioning that the Hans form is the first bioinnovative drug developed by Fosun Pharmaceutical. It is also the world's first anti-PD-1 monoclonal antibody for first-line treatment of small cell lung cancer (SCLC). Since it was approved for domestic marketing in 2022, it has now covered four indications. The fifth indication for first-line first-line treatment of non-squamous non-small cell lung cancer (nsNSCLC) has also been accepted by the State Drug Administration. It is expected to be approved domestically in the second half of this year.
Wu Yifang revealed that since the development of Fosun Pharmaceutical, the contribution to the company of creating varieties worth 200 million yuan has been very limited. “Our important task in the future is to focus on real high-value clinical products and create large varieties over 1 billion yuan.” He believes that although many products, including Hans Type and Han Quyou, are still in the process of being expanded recently, they all have the potential to become large single products worth 1 billion yuan or more in the future.
Referring to Fu Hong Han Lin, Wu Yifang also revealed the relevant progress of his privatization at the performance conference. “We are proceeding normally with Fuhong Hanlin's privatization. Regardless, our strategic goal is very clear, which is to deeply integrate Fuhong Hanlin's entire business, pipeline, and Fosun Pharmaceuticals through privatization.”
“Currently, Fuhong Hanlin is mainly developing oncology drugs in the direction of ADC and monoclonal antibodies. Fosun Pharmaceutical has a wider range of indications in the field of oncology. After privatization and integration with the parent company, Fuhong Hanlin's scope in the field of oncology will also expand a lot.”
Wu Yifang also believes that privatization will help Fuhong Hanlin to better “go overseas.” “In the process of going overseas, the parent company has many capabilities to take the lead. These can empower Fuhong Hanlin. At the same time, Fuhong Hanlin also has many products with an international foundation, which is a good collaboration with Fosun Pharmaceutical's overseas business.”

According to reports, in the first half of the year, Fosun Pharmaceutical's revenue from overseas reached 5.51 billion yuan, up 15.13% year on year, accounting for 26.93% of revenue, an increase of 4.56 percentage points over the same period last year. Currently, Fosun Pharmaceuticals has an overseas commercialization team of nearly 1,000 people, covering overseas markets such as the US, Europe, and Africa.
Specifically, in the first half of the year, Han Quyou had 3 indications approved for marketing by the US FDA, covering the fields of breast cancer and stomach cancer, and became a domestic biosimilar approved in China, the European Union, and the United States. Hans also completed the first batch of overseas shipments in January 2024, becoming the first domestically produced PD-1 monoclonal antibody approved for marketing in Southeast Asian countries. Its marketing license application (MAA) in the European Union was also accepted by the European Medicines Agency (EMA) in March 2023.

 目前,按不同业务板块划分,复星医药的营收主要来自制药、医疗器械与医学诊断、医疗健康服务,制药板块仍是复星医药最大收入来源,该板块报告期内实现营收146.77亿元,同比减少8.24%,占营收比重为71.72%。
目前,按不同业务板块划分,复星医药的营收主要来自制药、医疗器械与医学诊断、医疗健康服务,制药板块仍是复星医药最大收入来源,该板块报告期内实现营收146.77亿元,同比减少8.24%,占营收比重为71.72%。