① Baike Biotech's performance in the first half of the year continued its positive growth since 2023, but compared to the same period last year and the first quarter of this year, the revenue and net profit growth rate declined markedly; ② Since 2024, at least 5 companies have submitted new drug marketing applications for the shingles vaccine. Baike Biotech reminds that it may gradually lose the first-mover advantage of the shingles vaccine in the future.
“Science and Technology Innovation Board Daily”, August 15 (Reporter Zheng Bingxun) Today, Baike Biotech (688276.SH) released its 2024 semi-annual report, achieving revenue of 0.618 billion yuan, an increase of 10.50% over the previous year, and net profit of 0.138 billion yuan to mother, an increase of 23.54% over the previous year.
The first half of the year's results continued the positive growth since 2023, but compared to the same period last year and the first quarter of this year, the growth rate of revenue and net profit declined markedly.
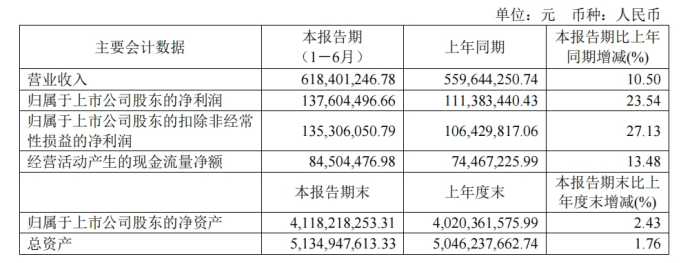
In the first half of 2023, Baike Biotech's revenue and net profit to mother were 0.56 billion yuan and 0.111 billion yuan respectively, with year-on-year growth rates of 26.93% and 51.39%. In the first quarter of this year, revenue and net profit to mother were 0.27 billion yuan and 60.5651 million yuan respectively, with year-on-year growth rates of 50.64% and 229.50% respectively.
Not long ago, in 2021 and 2022, Baike Biotech was also experiencing the “pain” of a continuous decline in revenue and net profit, and the launch of the shingles vaccine reversed this situation.
At the beginning of 2023, Baike Biotech's shingles vaccine obtained a “Drug Registration Certificate”, obtained a “Biological Product Batch Issuance Certificate” in April of the same year, and was successfully approved and sold in various regions. Together with the chickenpox vaccine and nasal spray influenza vaccine that had previously been approved, it formed Baig Biotech's commercial product line.
Moreover, as a latecomer, the shingles vaccine became Baig Biotech's most profitable core product once it was marketed. Taking 2023 as an example, the shingles vaccine achieved revenue of 0.883 billion yuan, accounting for 48.36% of overall revenue, higher than 44.92% of the chickenpox vaccine and 6.67% of the nasal spray influenza vaccine. The gross margin of the shingles vaccine was as high as 97.50%, 12.15 percentage points higher than the chickenpox vaccine, and nearly 27 percentage points higher than the nasal spray influenza vaccine.
Currently, in addition to 100g Biotech, GSK's shingles vaccine has also been approved for marketing in China. In the first quarter of this year, 25 batches of the shingles vaccine were issued, 12 batches of Baig Biotech's live attenuated herpes zoster vaccine, and 13 batches of GSK's recombinant shingles vaccine.
However, Baike Biotech also warned, “Currently, many domestic vaccine companies have deployed shingles vaccines. In the future, the company may gradually lose its first-mover advantage, which in turn will adversely affect the market share of the company's products.”
The “Science and Technology Innovation Board Daily” reporter's inquiry revealed that since 2024, there have been at least 5 companies that have submitted applications for the marketing of new shingles vaccine drugs, including Jia Chen Xihai (Hangzhou) Biology, Guangzhou Pinuo Biology, Jiangsu Zhonghui Yuantong, Yuanda Saiweixin, and Genovay (Shanghai) Biotech.
Compared to the competitive pattern of the shingles vaccine, Baig Biotech's nasal spray influenza vaccine may face greater competitive pressure. Since this year, leading domestic companies, including Sinopharm Group, Hualan Biotech, Kexon Biotech, and Jindick, have drastically lowered the price of the quadrivalent influenza vaccine.
Obviously, Baike Biotech is also concerned about this situation. It stated in its semi-annual report that some domestic vaccine manufacturers have lowered the prices of some non-immunization plan vaccines, increasing competitive pressure on vaccine products, which may lead to a decline in the market share and competitiveness of the company's already marketed products, which in turn will have a certain impact on the company's performance.
According to reports, Baig Biotech's chickenpox vaccine, shingles vaccine, and nasal spray influenza vaccine are all vaccines under the national non-immunization plan. Among them, the chickenpox vaccine is included in local immunization plans by some cities, and the non-immunization plan vaccines are priced independently according to the market.
The “Science and Technology Innovation Board Daily” reporter wrote to Baike Biotech about this, inquiring about the impact of the price reduction on the company's influenza vaccine. Baike Biotech did not respond directly, but only stated, “In response to the current price reduction situation of competitive products, the company will continue to monitor the market situation. If there are any future price changes, they will be promptly disclosed in accordance with relevant regulations.”
