Looking at the mid-year reports from CXO leaders such as WuXi AppTec, IQVIA, and Lonza, hand orders are exceeding expectations. Among them, WuXi AppTec's new hand order amount (excluding COVID-19 business) increased by 33.2%, far beyond the market expectations. The recovery trend of overseas biotech companies has already emerged.
$WUXI APPTEC (02359.HK)$The interim report was released and the full-year performance guidance has been maintained.
Overall, the impact of the Biosafety Act is clearly far less than the market's pessimistic expectations.
More noteworthy is that the interim report hides a more important trend, the obvious recovery of overseas biomedical industry. As an industry leader, Wuxi Apptec is expected to benefit greatly from the industry's recovery and maintain its industry-leading advantage.
1. Wuxi Apptec's Q2 report, new orders exceeded expectations.
In terms of Wuxi Apptec's Q2 2024 performance, revenue increased by 16% month-on-month to 9.26 billion yuan, consistent with market expectations, and consistent with the performance forecast previously released by Pharmaron and Asymchem Laboratories.
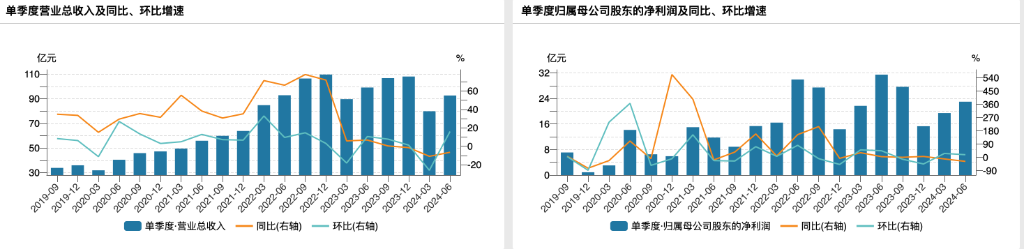
It is worth noting that Wuxi Apptec's month-on-month growth rate is still higher than Pharmaon's 4.8%-11.2% and Asymchem Laboratories' slight growth.
Wuxi Apptec's adjusted non-IFRS net income attributable to its parent company increased by 28.5% month-on-month to 2.46 billion yuan, higher than its revenue growth, mainly due to better cost control.
Due to the characteristics of the CXO industry, the conversion of orders in hand into performance is relatively predictable, so this part is basically in line with expectations. Compared with the same period last year, the month-on-month growth rate in the second quarter has surpassed domestic counterparts, demonstrating the resilience of the industry leader.
However, the most eye-catching data this quarter, also the data that exceeded market expectations the most, comes from the increase in newly added orders (excluding new crown business), which grew by 33.2%. As of the end of June 2024, the company's orders in hand amounted to RMB 43.1 billion.
This data is surprising because even the most optimistic investors find it difficult to predict an increase in newly added order amounts of more than 10% under the current impact of the Biosafety Act.
According to the order funnel chart,
R (Research Services) orders increased by 7%
D (Development Services) orders increased by 18%
M (Manufacturing Services) orders increased by 20%
All three parts have a growth rate lower than the 33% growth rate of the amount of newly added orders, meaning that "high-priced large orders" still account for the majority of newly added orders.
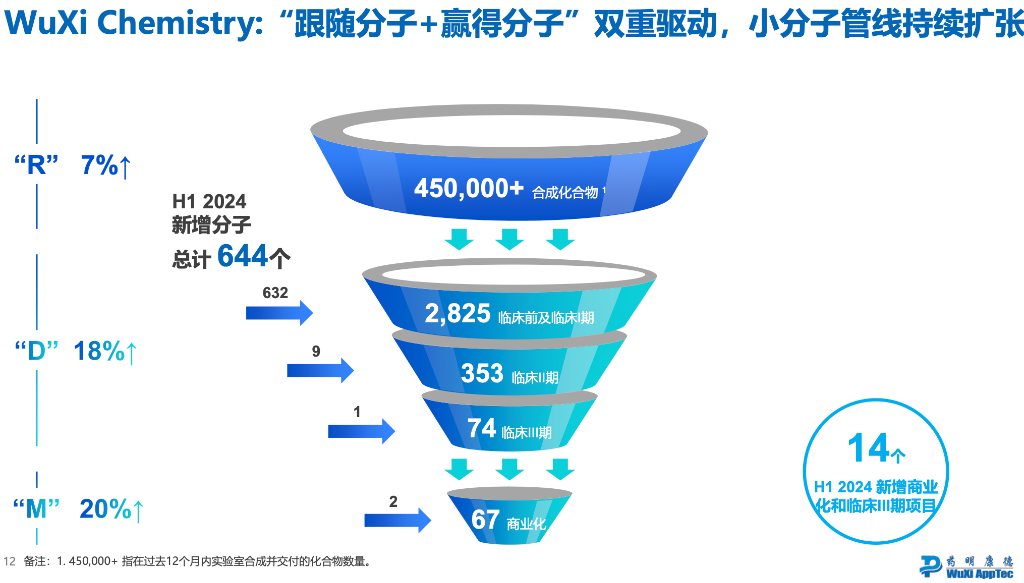
The company stated:"The relevant orders will be converted into performance within 18 months. At the same time, some customers have signed long-term agreements using grandfather clauses, which do not meet our definition of orders in hand and therefore are not included in orders in hand."
Regarding the full-year performance, the company further stated: This year's performance guidance remains unchanged, and the guidance for 2025 needs to pay attention to the growth of orders in the second half of this year, which will be disclosed in the annual report.
It is worth mentioning that the TIDES business (mainly including peptide drugs for weight loss) with the highest market attention continued to maintain high-speed growth in the second quarter.
At the end of the second quarter, the amount of orders in hand for this business increased by 147% year-on-year, with 288 services provided, a year-on-year increase of 39%.

Regarding the future prospect of the TIDES business, the company stated in its conference call: TIDES will become an important growth engine for the company in the future. It is expected to grow by more than 60% in 2024 and will maintain this growth rate in 2025.
In terms of production capacity planning, the company's production capacity in January of this year was 32000L, and it indicated that it will continue to increase investment to further expand its polypeptide production capacity to meet customer demand.
In addition, WuXi AppTec revealed that the revenue from the top 20 global pharmaceutical companies reached 6.59 billion yuan, with a year-on-year growth of 11.9% excluding the commercialization projects for the COVID-19 vaccine. Against the backdrop of major clients adjusting their research and development pipeline priorities due to the impact of the Inflation Reduction Act (IRA), this growth demonstrates the company's business resilience.

At the same time, overseas markets, especially the European market, contributed significantly to the increase.
2. WuXi AppTec's performance confirms the trend of industry recovery.
It is worth noting that it is not just WuXi AppTec that has reported better-than-expected growth in orders in the second quarter. As previously announced, Asymchem Laboratories and Pharmaron have also provided data on orders growth that exceeded expectations.
Pharmaron disclosed in its performance forecast:
"By the first half of 2024, the newly-signed order amount will increase by more than 15% year-on-year, of which laboratory services will increase by more than 10%, CDMO will increase by 20-30%, clinical research services will increase by 10%, and large molecule CDMO will increase by more than 10%."
Asymchem Laboratories disclosed in its announcement:
"By the first half of 2024, newly-signed orders will increase by more than 20% year-on-year, and the growth rate of customer orders from European and American markets will exceed the company's overall order growth rate in the second quarter compared to the first quarter."
Especially when Asymchem Laboratories directly pointed out that the volume of orders from European and American customers exceeded the overall growth level of the company, it confirms the trend of recovery in overseas pharmaceutical markets.
According to the latest pharmaceutical financing data from Jefferies, despite the negative impact of refinancing (FO) dragging down the overall month-on-month growth, the second quarter still achieved a 35% year-on-year growth. What is more notable is that the financing amount of US biotech companies is expected to increase by a large margin of 72% year-on-year in the first half of 2024.

While US interest rates remain high, the biotechnology financing market has gradually returned to pre-tightening levels. Once the US market achieves interest rate reductions in September, biotechnology financing is expected to further increase.
The significant recovery of the biotechnology financing market has also driven the order volume of the CXO industry. Not only have the aforementioned three Chinese companies reported better-than-expected orders, overseas giants have also disclosed positive order signals brought about by the industry's recovery in their recent mid-year reports.
Lonza stated in its semi-annual report that due to the improved financing environment for the biopharmaceutical markets in the US and Europe, the company's biologic CDMO business performance exceeded expectations (with a growth rate of 2%), and the company's core EBITDA exceeded expectations (with a growth rate of 6%).
Moreover, in the subsequent conference call, Lonza expressed even greater optimism, stating that there had been more requests for early-stage proposals (RFPs) in the second quarter, which are attributed to the recovery of the industry, rather than the spill-over effects of the Biologic Safety Act.
"There has been a significant increase in demand for early-stage RFPs. We believe this is largely attributable to the improved financial conditions in the biotechnology sector, rather than the impact of the Biologic Safety Act. Compared to the same period last year, the financial conditions of biotechnology companies have improved significantly, with funds almost increasing by 30% in the first half of the year. Thus, the market environment has undergone a significant change."
Lonza further stated that it takes 6-9 months for these funds to be translated into the company's orders. Moreover, CDMO will not see a price decline this year.
Another industry giant, IQVIA, also set a new record for backlogged orders in the second quarter, with all prospective indicators showing a growth trend.
As of the end of the second quarter of 2024, the company's backlog order amount reached US$30.6 billion (with a year-on-year growth of 7.7%, adjusted for exchange rates; 8.1%, unadjusted for exchange rates).
Both IQVIA and Lonza share the view that the biotechnology financing environment has improved.
IQVIA said:
The biotech financing amount in the first half of 2024 is about $70 billion, almost equivalent to the sum of the entire 2023, which is undoubtedly a positive signal for the company's order growth. However, IQVIA also pointed out another trend in the pharmaceutical industry: large pharmaceutical companies are adjusting their project portfolios, reducing cost expenditures, and focusing funds on the most attractive projects in response to the impact of the Inflation Reduction Act (IRA). This may bring more order opportunities for large CXO companies, and outsourcing is expected to help large pharmaceutical companies reduce costs, but attention needs to be paid to possible price competition. IQVIA said it will use AI automation and other methods to further reduce costs and increase efficiency to gain a competitive advantage.
Danaher expressed the view from the perspective of production capacity that demand will continue to increase: the market's capacity, especially the commercial production and clinical phase III capacity, needs to be increased; in the long run, the capacity of large pharmaceutical companies or CDAMs is insufficient, and we are optimistic about the growth of equipment orders.
This may bring more order opportunities for large CXO companies, and outsourcing is expected to help large pharmaceutical companies reduce costs, but attention needs to be paid to possible price competition.
IQVIA said it will use AI automation and other methods to further reduce costs and increase efficiency to gain a competitive advantage.
Thermo Fisher Scientific also stated in its Q2 conference call that bio-tech customers are optimistic about funding in the first half of this year, which will translate into early order indicators for Thermo Fisher and is expected to continue to improve in the second half of the year. Major customers are indeed concerned about supply chain flexibility and hope to stabilize product delivery.
Thermo Fisher Scientific also stated in its Q2 conference call that bio-tech customers are optimistic about funding in the first half of this year, which will translate into early order indicators for Thermo Fisher and is expected to continue to improve in the second half of the year. Major customers are indeed concerned about supply chain flexibility and hope to stabilize product delivery.
Thermo Fisher Scientific also stated in its Q2 conference call that bio-tech customers are optimistic about funding in the first half of this year, which will translate into early order indicators for Thermo Fisher and is expected to continue to improve in the second half of the year. Major customers are indeed concerned about supply chain flexibility and hope to stabilize product delivery.
In the background of the Biological Safety Act, the chemical business orders of Wuxi AppTec maintained a growth trend in the second quarter, and TIDES and small molecule DM continue to grow well, mainly because customers' demand for high-quality and compliant production capacity continues to grow, which is also the company's certainty competitive advantage in uncertainty. Currently, testing and biology sectors are mainly affected by prices, with only a very small proportion of early-stage research and development affected, and the proportion of orders contributed by Europe and the United States is similar to that in the past, with no special changes. The most severely affected is the ATU department (mainly involving cell and gene therapy), which is also the part of the company's demonstration material that was not as expected due to the impact of the law on revenue and profits. Due to its special product requirements, customer concerns are the highest, and the maximum limit for adding new products is limited. Currently, the company will work hard to complete the orders in hand.
Significant differences in the impact of the Biological Safety Act on different business sectors.
In its financial report conference call, Wuxi AppTec also expressed the concern of large customers about supply chain flexibility, which is similar to what Thermo Fisher Scientific expressed in its conference call.
In the background of the Biological Safety Act, the chemical business orders of Wuxi AppTec maintained a growth trend in the second quarter, and TIDES and small molecule DM continue to grow well, mainly because customers' demand for high-quality and compliant production capacity continues to grow, which is also the company's certainty competitive advantage in uncertainty.
Currently, testing and biology sectors are mainly affected by prices, with only a very small proportion of early-stage research and development affected, and the proportion of orders contributed by Europe and the United States is similar to that in the past, with no special changes.

The most severely affected is the ATU department (mainly involving cell and gene therapy), which is also the part of the company's demonstration material that was not as expected due to the impact of the law on revenue and profits. Due to its special product requirements, customer concerns are the highest, and the maximum limit for adding new products is limited. Currently, the company will work hard to complete the orders in hand.
It's worth mentioning that at the end of the conference call, some analysts optimistically asked whether the company will raise this year's guidance, reflecting some changes in the previously extremely pessimistic market expectations. The company's response was confident of meeting full-year guidance this year, with a delivery coverage rate of 85%, which is basically the same as in previous years. Next year's performance guidance will be disclosed in the annual report based on the order situation in the second half of the year.
It's worth mentioning that at the end of the conference call, some analysts optimistically asked whether the company will raise this year's guidance, reflecting some changes in the previously extremely pessimistic market expectations. The company's response was confident of meeting full-year guidance this year, with a delivery coverage rate of 85%, which is basically the same as in previous years. Next year's performance guidance will be disclosed in the annual report based on the order situation in the second half of the year.
Summary
From the performance of global CXO industry leaders, the industry has shown some positive changes. After the biotech financing environment improves in the first half of 2024, biotech companies are expected to gradually increase capital expenditures in the next 18 months, which will further promote the performance of other companies in the industry chain.
This is also a change that biomedical investors need to continue to pay attention to in 2024.
Editor/Lambor
