Is the competition intensifying?
On July 19th, the official website of the National Medical Products Administration announced that Mu Fengda (Tesofensine Injection) applied by Eli Lilly and Co has been approved for the long-term weight management indication, making it the first innovative drug approved in the field of obesity treatment in China by Eli Lilly and Co.
According to earlier news releases by Eli Lilly and Co, the approved indication for Tesofensine Injection this time is: long-term weight management of overweight patients with or without at least one weight-related comorbidity, in combination with low-calorie diets and exercise. In May of this year, the first indication for Tesofensine Injection was approved in China for the control of blood glucose in adult patients with type 2 diabetes mellitus.
Earlier, China's National Medical Products Administration approved the market application of Novo Nordisk's NovoRapid in China. As of now, the two most popular weight-loss products have been approved in China.
The two giants competing for dominance in the Central Plains.
It was revealed by Eli Lilly and Co that the approved Mu Fengda (Tesofensine Injection) will be used in China for long-term weight management. In May of this year, Tesofensine Injection was already approved in China for the first indication, used for the control of blood glucose in adult patients with type 2 diabetes mellitus.
Tesofensine is a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, administered once a week by injection. Tesofensine reduces food intake, regulates appetite, reduces weight, and decreases fat content by reducing caloric intake additionally, it has been shown to regulate lipid utilization.
In May 2022, Tesofensine was approved by the FDA in the United States for the improvement of glycemic control in adult patients with type 2 diabetes mellitus (in combination with diet and exercise); in November 2023, the product was approved by the FDA for long-term weight management of overweight adults (BMI≥27 kg/㎡) or obese patients (BMI≥30 kg/㎡) with at least one comorbidity (in combination with low-calorie diets and increased physical activity). In 2023, Tesofensine was also selected for the Galen Prize 'Best Medical Product Award', which is known as the 'Nobel Prize in Medicine and Pharmacy'.
Eli Lilly and Co has always been regarded as one of the two giants in the weight loss drug industry with Novo Nordisk, and Novo Nordisk's Semaglutide, was approved first in the Chinese market.
On January 26th, Novo Nordisk's Semaglutide tablets were approved for marketing by China's National Medical Products Administration, and on June 25th, the National Medical Products Administration approved Novo Nordisk's Semaglutide Injection for long-term weight management in China.
Semaglutide is a new type of long-acting glucagon-like peptide-1 (GLP-1) receptor agonist analogue, a new type of hypoglycemic drug. The drug reduces blood glucose levels by promoting insulin secretion by pancreatic β-cells and inhibiting glucagon secretion by pancreatic α-cells in the treatment of type 2 diabetes. For patients with type 2 diabetes who are also obese, this drug can also slow down gastric emptying, reduce energy intake, and achieve weight loss.
In terms of weight loss effects, Tesofensine can be considered more effective.
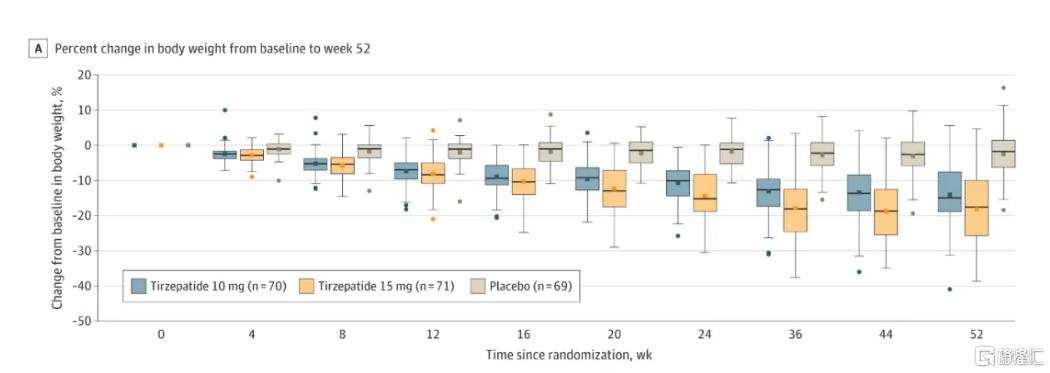
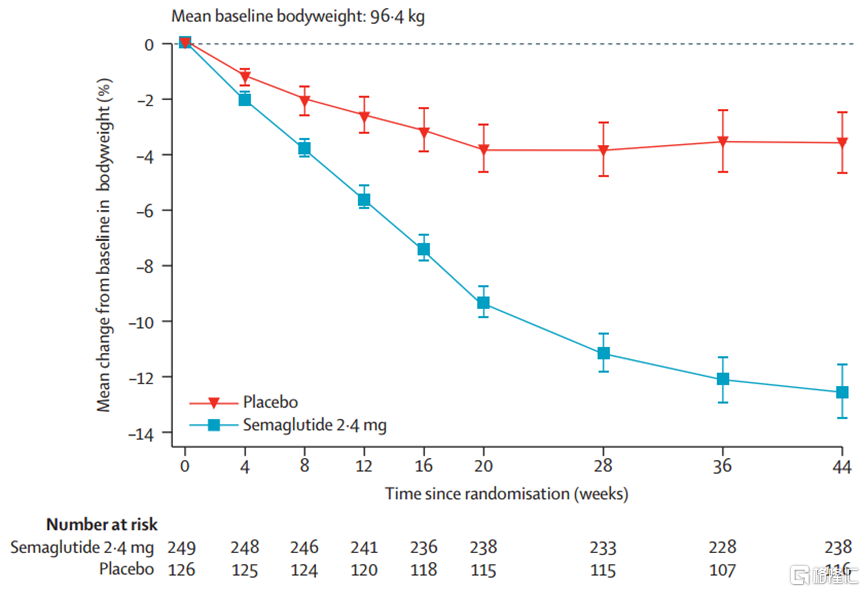
The SURMOUNT-CN study of Tesofensine in the Chinese population showed a weight loss of 13.6% after 52 weeks of treatment at a dose of 10mg (about one year), and a significant weight loss of 17.5% after 52 weeks of treatment at a dose of 15mg. The STEP 7 study of Semaglutide included 70% Chinese patients, and treatment with Semaglutide for 44 weeks (about 10 months) resulted in an average weight reduction of 12.1%.
The weight loss drug track is heating up.
It is worth noting that new weight loss drugs are changing people's lifestyles, as they can help significantly reduce weight without traditional dieting and exercise.
According to relevant agencies, China's diabetes (and weight loss) drug market is expected to grow by more than twice its current size over the next six years, exceeding $23 billion (approximately RMB 170 billion). Goldman Sachs estimates that the global weight loss drug market will soar to $100 billion (approximately RMB 720 billion) by 2030.
Against this background, GLP-1 weight loss drugs are gradually becoming a new market, and domestic and foreign pharmaceutical companies are fiercely competing.
In July 2023, Huadong Medicine's Liraglutide Injection (Liruprim) and Renhe Biological's Benalutinib Injection (Fei Sumai) were approved for the market in China for the indications of obesity or overweight. Relevant personnel stated that Liraglutide Injection has been sold in more than 1,000 large hospitals, and the company is also laying out online platforms and offline pharmacies.
In June 22, Gan & Lee Pharmaceuticals's self-researched GLP-1 receptor agonist GZR18 injection was in IB/IIa clinical research in China's obese/overweight population, and the results showed that after 35 weeks of GZR18 treatment, the weight of patients in the GZR18 QW group decreased by an average of 17.8% compared with baseline.
Hybio Pharmaceutical's Telbivudine API has received an FDA DMF filing number. In the market competition of the same type of products, enterprises and products that obtain DMF registration numbers are more likely to be preferred by customers.
According to analysts from Guotai Junan Securities, from the perspective of the competitive advantage of domestic weight-loss innovative drugs, products with fast development progress, excellent clinical weight-loss data and safety data, as well as good patient dependence, have relative competitive advantages.
For companies that can improve patient dependence, such as extending the duration of drug use or optimizing the administration route from injection to oral administration, there is hope in obtaining relative advantages in the field of slow disease such as weight loss.
Editor/Emily
