Source: Jinduan. Author: Lin Yao Shi. Twelve years ago, the world's most powerful orthopedic giant was born, and since then the entire industry has been mired in endless internal rectification. In April 2011, the world's largest orthopedic company, orthopedics company announced a record-breaking $21.3 billion acquisition of another orthopedic giant Synthes. At that time, Johnson & Johnson, with its previous acquisition of DePuy, became the global leader in the orthopedic business market share, but only had a gap in trauma products, occupying only about 5% of the market share. Swiss medical device company Synthes, although its revenue is less than that of Johnson & Johnson, is the strongest competitor in the trauma product industry, with a 49% market share. The historical largest merger and acquisition transaction enabled Johnson & Johnson Orthopedics to successfully acquire Synthes and become the dominant orthopedic giant in the market with no shortcomings in the joint, spine, and trauma submarkets. Other orthopedic companies were only stuck in the internal rectification in front of Johnson & Johnson Orthopedics' absolute market share. However, the orthopedic market story did not end here.
Author: QING LI.
Everyone knows about "medicine", it started with GLP-1, but few people know that the research on Alzheimer's disease (AD) also contributed to a significant market valuation of eli lilly and co with a market cap of 800 billion USD. The main reason why Eli Lilly and Co. is able to lead in market cap compared to Novo Nordisk A/S, besides the accelerated output of the magic medicine Tirzepatide, is due to its continuous research in the AD field. In fact, whenever Eli Lilly has made breakthroughs in the AD field over the past five years, it has boosted the rise in the company's stock price.
On July 2, the Donanemab developed by Eli Lilly, which is called Kisunla, has been approved by the FDA for the treatment of early symptomatic Alzheimer's disease, officially entering the door of the billion-dollar potential market in AD, and will confront Bojiang positively. Eli Lilly has finally wielded its "Second Sword".
The weather is good today The weather is good today.$Eli Lilly and Co (LLY.US)$Please use your Futubull account to access the feature.
Eli Lilly and Co. is the most persistent out of numerous pharmaceutical companies in putting effort to study AD drug development since the 1990s. The reason why it is able to lead in market cap compared to Novo Nordisk A/S, in addition to the acceleration in the output of weight loss wonder drug Tirzepatide, is due to long-term continuous research in the field of AD. In the past five years, whenever Eli Lilly has made breakthroughs in the AD field, it has boosted the rise in the company's stock price. On July 2, the Alzheimer's drug Donanemab (Kisunla), developed by Eli Lilly, was approved by the FDA for the treatment of early symptomatic Alzheimer's disease, officially entering the door of the billion-dollar potential market in AD and will confront Bojiang positively. Eli Lilly has finally wielded its "second sword".
On July 2, the Alzheimer's drug Donanemab (Kisunla), developed by Eli Lilly, was approved by the FDA for the treatment of early symptomatic Alzheimer's disease, officially entering the door of the billion-dollar potential market in AD and will confront Bojiang positively. Eli Lilly has finally wielded its "second sword".
01 Knights who challenge windmills.
Eli Lilly, laying out its plans for AD, was once seen as a "Don Quixote"-like act.
Although AD has been discovered for over a century, humans have been unable to find a way to conquer it. Currently, there are about 50 million AD patients worldwide, and with the aging of the global population intensifying, it is estimated that this number will reach 0.13 billion by 2050. With increasing age, the probability of suffering from AD becomes higher and the probability of an 85-year-old person having AD can exceed 30%. It can be said that AD is a disease that is closely related to each of us.
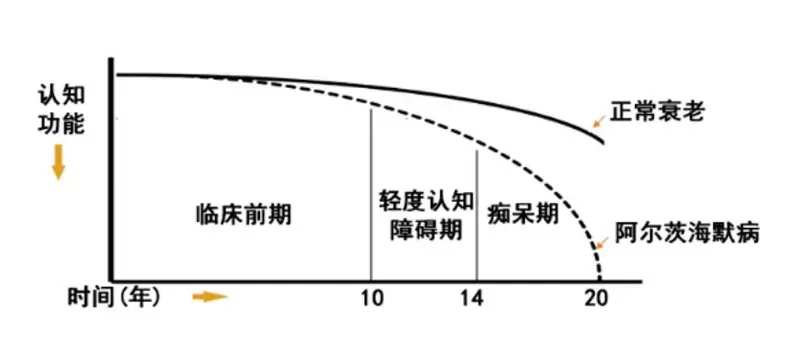
Before 2021, the FDA in the United States had only approved six drugs for the treatment of AD, and these drugs could only improve patients' symptoms, not alleviate the pathological changes of AD or reverse or slow down the disease process that has occurred, and these drugs have many side effects.
The huge user base and mysterious pathogenesis have aroused the ambition of countless scientists, making conquering AD an extremely important issue in the pharmaceutical industry. In order to find the answers, numerous pharmaceutical companies have invested a lot of time and money, but have always failed to reap results, and even have difficulty grasping its pathogenesis theory, hence AD was once referred to as the "RD black hole" of pharmaceutical companies.
Eli Lilly, which is the most tenacious, began to delve into AD drug development as early as the 1990s.
In 1992, British scientist John Hardy published the "Aβ cascade hypothesis" in Science. This hypothesis suggests that Aβ deposition is the main triggering factor for a series of molecular events that lead to the production of over-phosphorylated tau protein oligomers, which in turn activate the mechanism of neuron apoptosis and eventually lead to AD. Under this theoretical system, "Aβ is the killer, tau is the smoke gun, and neurons are the victims".
The "Aβ cascade hypothesis" provided a new idea for AD drug development. Eli Lilly scientists designed a monoclonal antibody against soluble β-amyloid protein according to this hypothesis, Solanezumab, and published their research results in Nature Neuroscience in 2002. However, after a decade of long clinical trials, the drug was proven ineffective in 2012, and finally fell in the phase III clinical trial.
In the face of failure, Eli Lilly did not completely lose confidence. They believed that Solanezumab's mechanism was not the problem. Although it could not treat mild to moderate AD patients, it might be effective for early-stage AD patients. Therefore, Eli Lilly launched a new clinical study to test Solanezumab in patients with early AD who had already developed β-amyloid protein plaques but had not yet shown symptoms. A total of 1,100 patients received Solanezumab or placebo treatment for 4.5 years.
Reality is always cruel. In November 2016, Eli Lilly announced the clinical trial results: Solanezumab did not achieve the expected ideal effect and the trial eventually failed. By this point, Eli Lilly had invested over 3 billion USD in Solanezumab, but received nothing in return. After the news came out, the stock price of Eli Lilly plummeted by 16% on the same day. In addition, at that time, the core insulin sales of Eli Lilly were declining, and there were even rumors in the market that Eli Lilly would be acquired by other giants.
Solanezumab is just a microcosm of the failure of numerous AD drug developments. According to the research report released by the American Pharmaceutical Manufacturing and Research Association, more than 600 billion USD has been invested globally in AD research and development from 2000 to 2017, and more than 300 clinical drugs have failed, with a failure rate as high as 99.6%, including multinational giants such as Merck, Pfizer and Roche.
In the face of the "research and development black hole" AD, Eli Lilly accepted Solanezumab's failure calmly, but did not give up the research and development of this disease. Before Donanemab was approved, Eli Lilly had invested more than 8 billion U.S. dollars in R&D costs for AD indications. On the product structure, the operating income of 10-30 billion yuan products is 401/1288/60 million yuan respectively.
Eli Lilly's AD strategy: pipeline "horse racing"
Eli Lilly is well aware of the difficulties in the AD field, so it did not bet all its resources on Solanezumab, but adopted a matrix layout of product "horse racing".
In addition to Solanezumab, Eli Lilly also laid out several AD drugs such as Donanemab, Semagacestat, Lanabecestat and Remternetug. Although Semagacestat and Lanabecestat failed in 2010 and 2018 respectively, causing internal doubts in Eli Lilly on whether to persist in the "Aβ cascade hypothesis" route, it ultimately chose to persist. After learning from the failure experience, Eli Lilly shifted its research and development focus to antibodies that clear deposits, and Donanemab is such a product.
Ronald DeMattos, Ph.D. is a key figure in Eli Lilly's AD research and development system. He joined the company in 2002 and devoted himself to the development of Solanezumab. While developing Solanezumab, Dr. DeMattos began to research the "upgraded version" of Solanezumab. In the process of research, Dr. DeMattos discovered a specific cyclic structure N3pG in amyloid plaques, which provided a key target for drug targeted delivery, and the new antibody Donanemab was born.
Although Eli Lilly found the ultimate goal, the process of verifying Donanemab was still bumpy.
The preclinical stage went smoothly. DeMattos spent four years cultivating mice with plaque levels similar to the patient population. Donanemab successfully reduced the level of amyloid plaques in the mouse brain.
But in 2011, Donanemab entered clinical phase I, and data from the first phase almost caused the project to be terminated. The data showed that more than 90% of patients developed anti-drug antibodies against Donanemab. Researchers worried that this immune response would hinder the drug from entering the brain.
Fortunately, Eli Lilly detected deposition plaques through PET scans with radioactive molecules, and found that the drug was not affected by immune reactions. A single infusion of Donanemab could reduce plaque deposition in the brains of patients, and many patients who received multiple doses completely cleared plaques from their brains. This result greatly encouraged Eli Lilly's R&D team, and they began to push for the approval of Donanemab.
In January 2021, Eli Lilly announced that it had successfully completed Phase II clinical trials of Donanemab, and the results showed that Donanemab reduced the rate of cognitive and functional decline by 35% in some early-stage Alzheimer's disease patients within 18 months. However, because the Phase II clinical trial only enrolled 94 patients, and FDA specifically requested Eli Lilly to provide data from at least 100 patients who received 12 months of continuous treatment, Donanemab was refused early approval.
However, in January 2023, Orckonnetinib, a new drug for AD jointly developed by WCKN and Bao Wellbeing, was granted accelerated approval by the FDA and received full approval in July, becoming the world's first drug to delay the progress of AD. Although Eli Lilly failed to win in the "FIC" competition, "latecomer advantage" has always been its strategy.
In July 2023, Eli Lilly announced the latest complete Phase III clinical data of Donanemab. Compared with the placebo group, the cognitive and functional decline rate of early-stage patients slowed by 35% after treatment with Donanemab for 18 months. The effect was more significant in patients with mild cognitive impairment, with a slowdown of more than 50%, and 72% of patients could clear all plaques after using the drug for about a year.
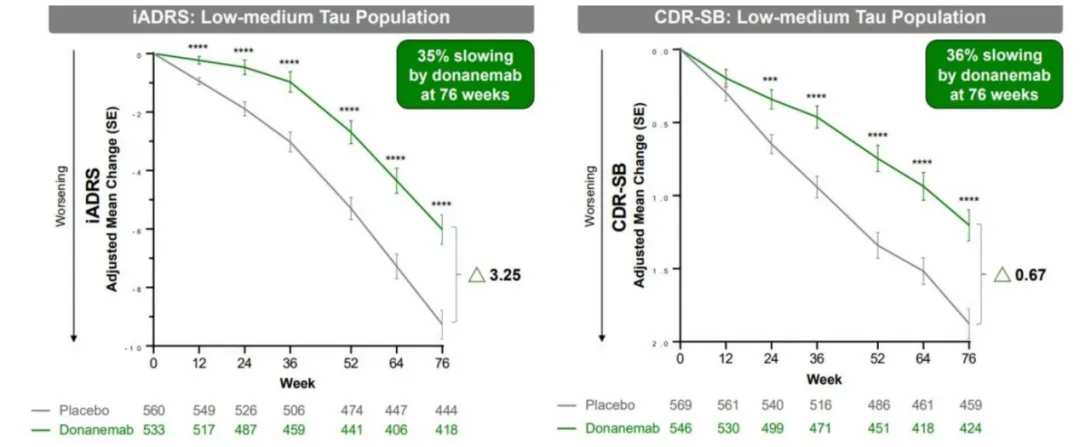
From the clinical research data, the efficacy of Donanemab is better than that of Leqembi. Leqembi reduced the rate of cognitive and functional decline in Alzheimer's disease patients by 27% within 18 months. Leqembi is given every two weeks, while Donanemab is given every four weeks.
Instead of saying that Donanemab is superior to Leqembi in terms of efficacy, it is better to say that Eli Lilly's preparation in the AD field is more adequate.
03 AD new king?
Based on excellent Phase III clinical data, Eli Lilly submitted Donanemab's marketing application to the FDA again in the second quarter of 2023.
The FDA was supposed to make a decision on the approval of the drug in the first quarter of this year. However, on March 8 this year, the FDA decided to postpone the approval of Donanemab, and held an external expert meeting to discuss the safety and efficacy of the drug, casting a shadow over the drug's approval. On that day, Eli Lilly's stock price fell by 2.31%. The phase III clinical data showed that 31.4% of treated patients had microbleeds in the brain, significantly higher than the 13.6% in the placebo group. In addition, 3 experimental patients died related to the treatment.
Until June 10 this year, the FDA's independent scientific advisory committee conducted a review and voted on two specific issues based on the results of phase II and phase III clinical trials. Ultimately, the committee unanimously approved its effectiveness with a vote of 11:0, and unanimously agreed that the benefits outweigh the risks. Thus, Eli Lilly finally sees the light at the end of the tunnel.
On July 2nd, Eli Lilly and Co announced that the new drug Donanemab has been approved by FDA for the treatment of early symptomatic AD, including patients with mild cognitive impairment (MCI) and patients with mild dementia stage of AD. The successful approval of Donanemab provides a new treatment option for AD patients, once again verifying the effectiveness of the Aβ hypothesis and inspiring AD drug projects under development, pointing the direction for future drugs based on beta-amyloid.
At the same time, the approval of Donanemab means that Eli Lilly and Co has finally gained a return on its continued investment in the AD field for more than 30 years. Compared with Leqembi, Donanemab is the first and only limited-course amyloid plaque targeted therapy. Patients can stop taking the medicine when they reach a certain level of amyloid plaque clearance rate, while Leqembi has no corresponding discontinuation plan. Currently, Donanemab is sold in the United States for $ 695.65 per bottle with a single-year treatment cost of 0.032 million US dollars; in contrast, the annual treatment cost of Leqembi is 0.0265 million US dollars.
In the first quarter of this year, Leqembi achieved a revenue of $19 million, far higher than the average analyst expectation of $7 million. This drug may become the revenue pillar of Eli Lilly and Co in the future. Donanemab, with better data, undoubtedly has greater potential and may even become a king of drugs, most likely becoming the next hot product similar to GLP-1.
More importantly, Donanemab is only the first step for Eli Lilly and Co to enter the AD field, and many other matrix products are still in progress. Eli Lilly and Co will not be satisfied with temporary victories and will continue to upgrade and iterate its products.
Remternetug is another AD drug developed by Eli Lilly and Co, the next generation of N3pG amyloid protein antibodies, which uses flexible administration methods such as intravenous injection and subcutaneous administration. The results of phase I clinical trials show that Remternetug can quickly clear plaques. After 169 days of administration, 18 of the 24 subjects achieved amyloid clearance, and the phase III trial is expected to be completed in October 2025.
Today, Eli Lilly and Co, the "King of Drugs", already has two swords in its hand. One is the GLP-1 drug, which has killed the four sides in the fields of diabetes and weight loss, making it difficult for latercomers to surpass. The other is the AD treatment drug, which has been painstakingly polished for more than 30 years. At the moment the sword is unsheathed, it is difficult for anyone to shake Eli Lilly and Co's position as the King of Medicine in the short term.
Editor/Somer
