Hidden risk of rare blindness.
Doctors at Massachusetts Eye and Ear Hospital, affiliated with Harvard Medical School, found that novo-nordisk a/s's popular weight loss drugs, Ozempic and Wegovy, appear to increase the risk of rare vision loss.
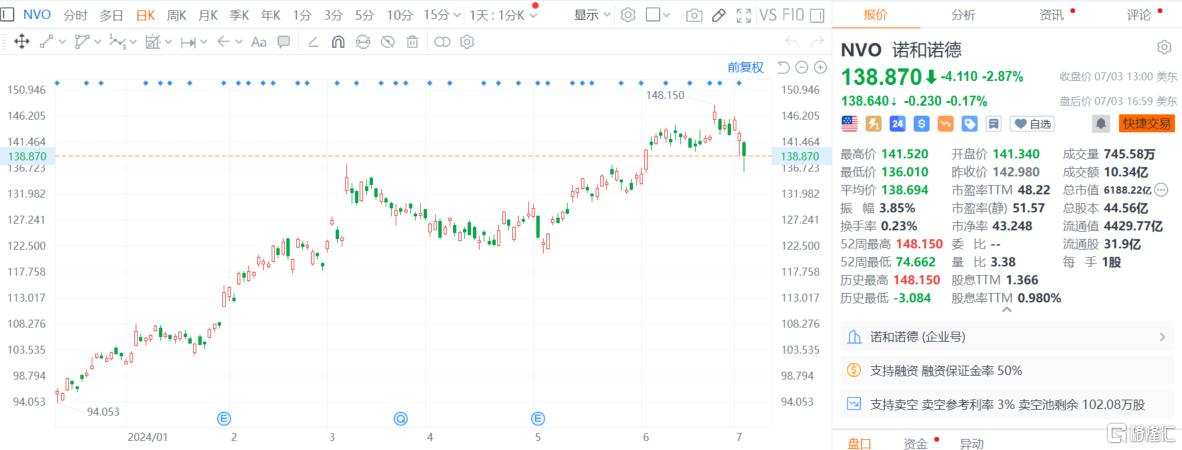
Affected by the news, novo-nordisk a/s's stock fell by 2.87% on Wednesday, and fell nearly 5% at one point during the day; eli lilly and co's stock fell by 0.95%, and its intra-day decline once exceeded 2%.
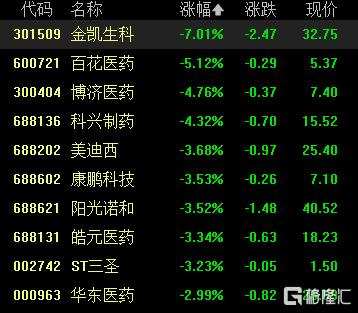
Today, stocks in the weight loss drugs sector fell across the board, with Jinkai Biological Engineering falling by over 6%, Xinjiang Bai Hua Cun Pharma Tech falling by over 5%, Boji Medical Technology and Kexing Biopharm both falling by over 4%, and Sunshine Novo Nordisk and Huadong Medicine following suit.
Ozempic and Wegovy are associated with rare vision loss.
According to an analysis by doctors at Massachusetts Eye and Ear Hospital, affiliateof Harvard Medical School, novo-nordisk a/s's best-selling diabetes and weight loss drugs, Ozempic and Wegovy, appear to be associated with an increased risk of a rare form of vision loss.
According to patient record studies, compared to those using other brand weight loss drugs, patients who use novo-nordisk a/s's weight loss drugs to lose weight are... ...seven times more likely to suffer from non-arteritic anterior ischemic optic neuropathy (NAION). Patients who take diabetes drugs are more than four times more likely to develop this rare disease than patients receiving other types of treatment.
Non-arteritic anterior ischemic optic neuropathy (NAION) is a relatively rare disease, affecting about 10 people per 100,000 in the general population. NAION is the second leading cause of optic nerve blindness (second only to glaucoma) and the most common cause of sudden optic nerve blindness.
The study states that there were relatively few NAION cases among patients taking these drugs (a total of 37 cases in both groups), which limited the study's statistical power.
Researchers at Harvard University and other experts said that these findings do not prove that these drugs cause eye complications, and that the results must be repeated in larger studies involving more hospitals. "I don't think this signal is strong enough to make patients stop taking the drug," said Susan Mollan, a neuro-ophthalmologist in Birmingham, United Kingdom, who wrote an editorial on the study. The study did not indicate that eye effects occurred immediately after taking the drugs, as some cases appeared months later. However, she said doctors should inform patients of potential risks.
Please use your Futubull account to access the feature.
