Source: Brocade
Author: Hayashi Pharmacist
In 1922, four researchers from Toronto successfully extracted insulin from animal pancreas, and since then humans have found a treatment plan for diabetes.
Although insulin at the time was already effective in lowering blood sugar in humans, since this type of insulin was extracted from the pancreas crushed by animals, the purity of the product was not high, and it was very easy to cause side effects such as itching and rashes all over the body. In severe cases, anaphylactic shock may even occur.
In order to reduce the side effects of animal insulin products, insulin manufacturers, led by Eli Lilly, began a 60-year “purification” competition. In this competition,$Eli Lilly and Co (LLY.US)$,$Sanofi (SNY.US)$Leading companies such as Novartis and Novartis continued to invest and eventually successfully reduced the amount of pollutants in insulin from the initial 50,000 ppm (ppm, that is, the amount of pollutants contained in each million volumes) to 10 ppm in 1980.
The insulin “purification” competition finally came to an end in 1982. Genetech, a “pioneer in genetic engineering technology,” successfully manufactured recombinant human insulin through Escherichia coli, and later licensed this technology to Eli Lilly. Since then, Eli Lilly has finally raised its insulin concentration to 100%, and everything indicates that it is about to win this “100-year battle” of insulin.
However, the direction of the story did not develop as Lilly had anticipated. Instead, in the process, it gave birth to its “lifelong enemy” — today's “European stock king”$Novo-Nordisk A/S (NVO.US)$.
01 Eli Lilly: Stuck in “continuous thinking”
Most technological changes have evolved along an established trajectory. For example, in the early days of insulin racing, continuous improvement in purity was the core path of technological change.
For top players such as Eli Lilly, as long as they can continue to produce more pure insulin products, the side effect rate can be reduced more significantly, thereby obtaining higher product premiums. It was through iterations of technology over and over again that Eli Lilly gradually built up a competitive advantage, which is why it is so obsessed with “purifying” insulin. In other words, early competition on the insulin circuit continued to develop along the line of improving purity.
Genetic-Tek cloned human insulin successfully solved the problem of animal insulin producing immune responses, with almost no side effects. It was a perfect product for diabetics at the time, but this perfect product didn't give Lilly enough in return.
In order to industrialize and mass-produce insulin eucillin, Eli Lilly invested nearly 1 billion US dollars in the 80s of the last century, hoping that this cross-era product would monopolize the entire insulin market. As a result, Eli Lilly's price for eucomulin was not high; the price increase was only 25% compared to conventional animal insulin.
However, the sales data for this product across the ages was poor, and Lilly even found that it was difficult to sell eubiline at a higher premium than animal insulin. Although in the end, human insulin completely replaced animal insulin, compared to Lilly's high investment in the early days, “innovation across the ages” clearly did not bring victory to Lilly in commercialization.
Analyzing the reasons why it is difficult for eubilin to form a premium is solidifying “continuous thinking.”
In the early days of industrial development, due to the extremely high percentage of pollutants in animal insulin, purity was the most important concern for diabetics. As long as manufacturers continue on this path of technological change, they can easily achieve commercial success.
However, after all, there is an upper limit for “purifying” insulin. As technology continues to improve, the industry is getting closer to this upper limit. By 1980, the purity of insulin had increased to 10 ppm, and this purity had reached almost 100%. There was nothing wrong with Lilly's obsession with perfect insulin products at this time, but purity was no longer the core of insulin competition.
Although animal insulin still causes an immune response in some diabetics, the rate of side effects has been significantly reduced compared to the initial rate, and animal insulin can already meet the needs of most diabetics. On this basis, only a small number of patients with an immune response will choose the more expensive human insulin, which in the eyes of most patients is more like an “IQ tax.”
Addicted to “continuity thinking,” he overlooked the main contradiction in insulin development, which is why Eli Lilly did not achieve the expected success at the time. The “primacy” of industrial development is not immutable; it is constantly evolving along with technological changes.
02 Novo Nordisk: Overtaking cars that fill clinical gaps
Since Lilly misestimated the “primacy” of insulin development in the 80s, what exactly were the core pain points of diabetics at the time? It is clinical compliance that does not meet the requirements.
Although insulin was already able to meet the hypoglycemic needs of most diabetics at the time, injecting insulin every day was still quite complicated. Diabetics can only fill the syringe with insulin for a day at a time. Each injection needs to squeeze out the bubbles, and also control the amount of each injection. This makes a simple insulin injection often take several minutes.
It may be difficult for people today to sense the pain of diabetics back then. This was not only a physical pain, but also a mental pain caused by careful insulin injections every day. This nuanced slice of clinical needs was accurately observed by Novartis of Denmark, so when Eli Lilly promoted human insulin, Novartis (the predecessor of Novo Nordisk) was trying to compete for the market by designing a more convenient syringe.
In 1986, when Eli Lilly's insulin sales fell short of expectations, Novartis successfully developed a new generation of insulin injections — Novo needles. The Novo needle is equivalent to a specially designed pen and contains several weeks of insulin. When injecting insulin, diabetics only need to press the injection button to complete the insulin injection within 10 seconds.

Unlike Eli Lilly, which painstakingly supports the 25% premium rate of eubulin, Novartis achieved a sales premium of 30% per unit of insulin, directly increasing Novartis's share of the insulin market.
Thanks to the success of Novo Needle, Novo successfully acquired NORD, also from Denmark, two years later, and launched the world's first pre-filled insulin syringe, NovoLet in 1989. Relying on the high level of convenience of Novo Needle, the merged Novo Nordisk occupied 54.4% of the global market share, and overtook Novo Nordisk to become number one in the world.
The Novo needle was the first pot of gold discovered by Novo Nordisk. This also laid down Novo Nordisk's future development strategy close to clinical needs.
03 How can small companies beat Big Mac?
Compared to Lilly, which successfully snatched Genetec insulin, Novartis can only be considered a small company. However, it was this small company that successfully seized the opportunity of Lilly's strategic mistake and finally achieved a counterattack in market share.
Whether at the brand level, technical level, or capital level, Eli Lilly was the absolute brother on the insulin circuit at the time. Why did such a large company fail? This is actually a necessity.
In the early stages of industrial development, since there were no products that could meet user needs and functional requirements, the standard for industry competition was functionality, that is, the purity of insulin. However, when the purity of insulin is raised to a certain level, most mainstream insulin in the market can already meet the needs of users, so the competitive advantage of leading companies will decline.
When the performance of the product is sufficient, the improvement in performance will not bring the same competitiveness as before, and the focus of users' attention gradually shifts from functionality to other factors such as convenience and cost performance.
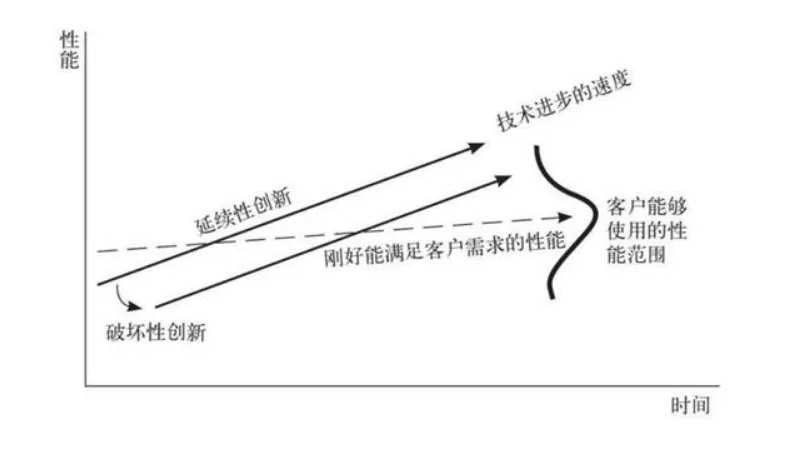
However, due to their long-standing industry leadership, leading companies have fallen into the misunderstanding that technology is king, which ultimately led them to invest more capital and energy into higher-performance technology routes. Perhaps such efforts will pay off at some point, but the industry will eventually change, and sooner or later large companies will suffer huge losses due to misplaced technology routes. However, players who are in a position to catch up in the industry are often more likely to perceive such changes, so there will always be classic cases of winning over strength by weakness in world business history.
Eli Lilly is not the first major company to be counterattacked, nor will it be the last. Large companies are inherently insensitive to the migration of technological change routes, and prefer to follow previous successful ideas. This is what is known as path dependency.
Thirty years later, insulin is no longer the core battleground of competition in the pharmaceutical industry, but Novo Nordisk, which focuses on unmet clinical needs, can still find new demand for weight loss in the hypoglycemic drug GLP-1.
In terms of GLP-1 weight loss indications, Novo Nordisk also showed pragmatism. After long-acting GLP-1 simeglutide met user needs, it began to focus on developing oral drugs to improve compliance; yet Lilly still seems to adhere to the perfectionist style of the year and pursue higher weight loss data, which certainly has the highest market value in the world at this stage, but there are still objective risks.
Only innovation can bring new opportunities, especially in the pharmaceutical industry, which continues to fill clinical gaps. Starting from clinical unmet needs, companies can truly seize opportunities in the industrial development process. It's just that too many large companies don't want to listen to outside voices, but rather indulge in grand narratives of their own.
Everything has a cycle, and there is a reason why prosperity and extreme decline are inevitable. If large companies want to maintain their strengths, they must abandon their position as big companies and continue to cultivate as industry explorers. For pharmaceutical companies, filling clinical gaps must be the most important, regardless of whether the path is improving efficacy, iterating technology, or improving compliance.
Editor/Somer
