June 21, 2024
NTT DATA CORPORATION
NTT DATA Corporation (hereafter: NTT DATA) collaborated with Kirin Breweries Co., Ltd. (hereafter: Kirin Breweries) to build a prescription management systemNote 1Then, full-scale operation of the system will begin in 2024/6.
In the food and beverage industry, improvements and sophistication of product development capabilities are required as consumer needs diversify and there is a shortage of human resources for product development. Kirin Breweries prescription development businessNote 2Then, since complicated prescription design, labeling studies, and multiple prototyping were necessary, the management and confirmation work of information was complicated, which became an issue.
NTT DATA is Kirin Brewery's RTDNote 3For products, we construct and provide a prescription management system that can centrally manage prescription development information as digital data, and by improving the efficiency of prescription development work, formalized knowledge of know-how, and improved work quality, approximately 2,000 hours per year together with Kirin BreweriesNote 4We aim to reduce work hours.
Going forward, by combining prescription development information accumulated as digital data with advanced technology such as machine learning/generative AI technology, we will support further improvement and sophistication of product development capabilities, create innovation for the food and beverage industry, and contribute to solving social issues.
backgrounds
In the food and beverage industry, as consumer needs diversify, product development in a faster cycle than before is required, and due to chronic labor shortages, securing and developing specialized human resources with advanced technology has become an issue for the entire industry. In Kirin Breweries's prescription development business, dozens of prescriptions are examined based on various types of raw materials, and prescriptions are determined through repeated prototyping and tasting, so utilizing and inheriting study information and know-how in prescription development work and improving work efficiency associated with it have become an issue.
NTT DATA has an alliance with the Kirin GroupNote 5Through this, we have contributed to the systematization of Kirin Breweries's entire value chain. The product development area is also highly complex, and while systematization has not progressed at an early stage until now, NTT DATA has been working on many advanced product development operations in the food and beverage industry with digital technology, and it has know-how and assets that can be transferred to Kirin Breweries issues and business areas this time.
Outline of efforts
NTT DATA has built a prescription management system that can centrally manage prescription development information as digital data for RTD products to solve Kirin Breweries issues, and after trial operation by some Kirin Breweries product teams, full-scale business operation of the system will begin in 2024/6.
Formulation development work is divided into formulation review work that examines raw materials and formulations used in new products, trial work that repeats prototyping/tasting based on examined prescriptions, cost review work that calculates costs, and labeling review work that examines raw materials/nutritional information labeling on product packages based on examined prescriptions. (Figure 1) In these operations, until now, they have been individually managed and operated with Excel forms, but by digitizing forms, data is centrally managed, and by systemizing complex business logic used in business (Figure 2), we support work efficiency, formal knowledge of know-how, and improvement of work quality, and aim to reduce work hours by approximately 2,000 hours per year. At Kirin Breweries, it is possible to further improve product development capabilities by devoting time created through this initiative to more advanced operations (raw material search, formulation review, technology search to develop better products).
1. Digitization of business forms and centralized management of data
Since forms were divided for each task of prescription review, prototyping, and labeling review, the prescription information (raw materials/formulation/manufacturing conditions) commonly used in each form was transcribed and confirmed on multiple forms every time a change was made, and a lot of operating time was spent. Also, not only are forms examined in the past managed closed to teams and individuals, but the layout of the forms was often changed, and past know-how could not be effectively utilized in new product development and development personnel training.
Now, prescription information is centrally managed as digital data, and by making it possible to use it as source data for prototyping and display studies, it is possible to shorten the time required for transcription and confirmation. Furthermore, since past prescription reviews, prototyping, and display study information can be viewed across teams and without depending on the layout, it is now possible to use it to pass on advanced technical knowledge.
2. Systematization of complex business logic
In formulation review work and trial work, accurate calculation and numerical data management is required for the raw materials to be handled, their formulation information, and prototype results. Also, when labeling raw materials and nutritional components on product packaging, it is required that reliable information be posted in accordance with the Food Labeling Act and industry voluntary standards. Until now, these have been operated with Excel forms, and it took a huge amount of time to confirm calculation results and numerical values and confirm the display.
Now, by systematizing these computational logic and business logic, we have realized an environment where logic errors are less likely to occur, contributing to shortening the time required for confirmation and improving the quality of product development work.
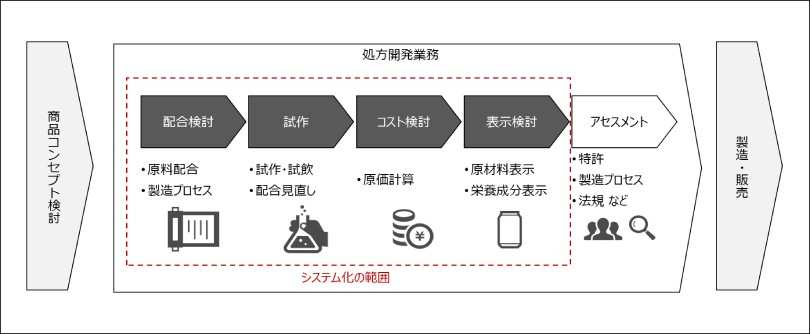
Figure 1: Scope of prescription development work and systematization
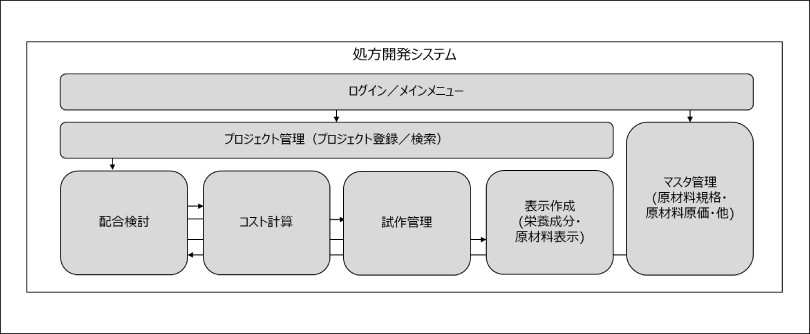
Figure 2: Functional image of a prescription management system
About the future
The digitalization of prescription development work, which has been realized this time, is the first step towards the advancement of future product development work. In the future, by combining accumulated data with advanced technology such as machine learning/generative AI technology, it can be expected to be used to predict optimal raw materials and formulations. Furthermore, by connecting upstream marketing operations and downstream production/sales operations with data, it is also possible to lead to business reforms across the value chain. NTT DATA will continue to utilize digital technology to create innovations leading to the sophistication of product development operations in the food and beverage industry and solutions to social issues.
notes
- Note 1 The prescription management system was constructed by NTT DATA BUSINESS SYSTEMS CORPORATION (https://www.nttdata-bizsys.co.jp/It is being carried out in collaboration with).
- Note 2 Formulation development business: A business that examines formulations (raw materials/formulation/manufacturing conditions) for new products, prototypes/tastings, and examines display information on product labels.
- Note 3 RTD: An abbreviation for “Ready to Drink.” It is an alcoholic beverage that can be drunk as is by opening the stopper.
- Note 4:2,000 hours per year: Target work reduction time due to the current systematization.
- Note 5 Alliance between NTT Data and Kirin Group: In 2012, NTT DATA took over 49% of the issued shares of Kirin Business Systems Co., Ltd., which is a wholly owned subsidiary of Kirin Holdings Co., Ltd., and began a capital alliance. (https://www.nttdata.com/global/ja/news/release/2012/030900/)
- Product names, company names, and organization names in the text are trademarks or registered trademarks of their respective companies.
Contact information for inquiries regarding this matter
NTT DATA CORPORATION
Second Industry Management Business Division
Food/Beverage/CPG Division
KIRIN Management Division Business Officer
Tanaka, Kameda
TEL:050-5546-9114
