① In order to avoid making the mistake of another “magic drug” decades ago, Eli Lilly is stepping up research on various forms of new GLP-1 drugs; ② In addition to oral medication, Lilly also has high hopes for the triple agonist drug retaglutide.
Finance Association, June 14 (Editor Shi Zhengcheng) At the beginning of 2018, Dave Rex, who had recently been promoted to CEO of Eli Lilly, was visiting the college campus with his daughter. At that time, he suddenly received a phone call from his deputy: The new drug tirpotide, which the company is testing, is not only useful for lowering sugar, but also helps with weight loss better than existing weight loss drugs.
(Dave Rex infographic, source: Eli Lilly's official website)
Standing on the UC Berkeley campus, Rex immediately ordered Eli Lilly to “go full speed.” As a result, Mounjaro, a type II diabetes drug with tiverpotide as the main active ingredient, was approved for marketing in 2022, and the diet drug Zepbound followed competitors Novartis and Nord to the market at the end of 2023.
Mounjaro and Zepbound generated a total of $5 billion in revenue in 2023. Driven by the “diet pill frenzy,” Eli Lilly's market value has tripled in the past two years. The latest figure has surpassed 800 billion US dollars. Not only is it the number one pharmaceutical company in the world, but it also ranks 10th among listed companies in the world.
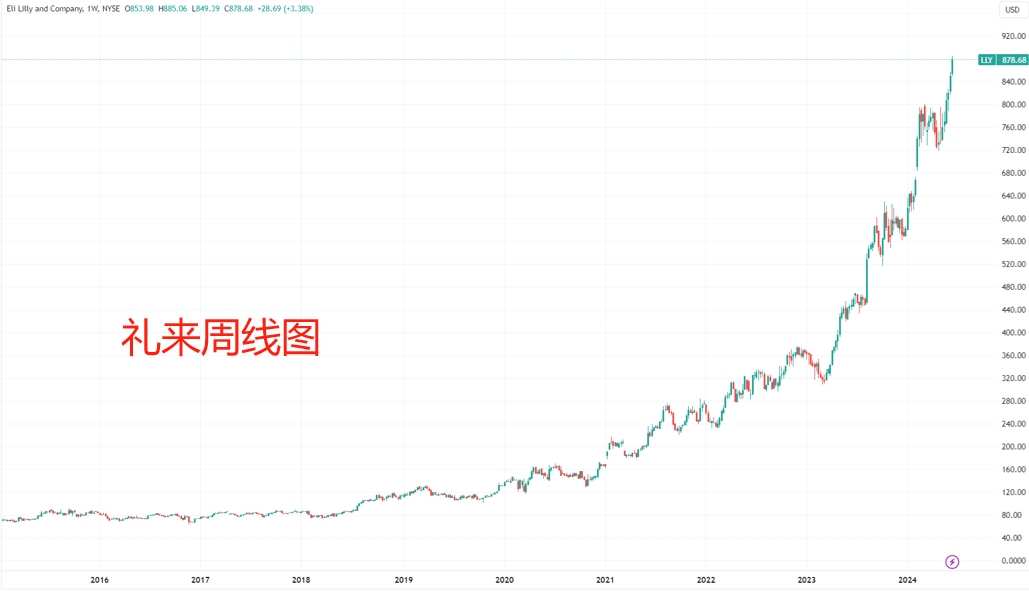
(Since that call, Eli Lilly's market value has increased tenfold, source: TradingView)
At a time when the company is fully coping with the shortage of tiverpopeptides production, as an industry leader, Rex is also under pressure from industry competition — can the company still stand at the head of the wave of technological iteration?
Have made mistakes
Lilly's anxiety isn't unfounded — the company experienced the glory and desolation of developing a “best-selling elixir” decades ago.
In the 80s of the last century, fluoxetine (also known as “Prozac”), an antidepressant developed by Eli Lilly, was called a “magic drug.” By the 90s, the annual revenue of this drug had reached 3 billion US dollars. But Lilly, steeped in the joy of success, did not continue to invest more in fighting depression.
By the time patent protection expired in 2001, the fluoxetine market was quickly occupied by generic drugs. Naturally, Lilly had no preparation for the next generation of products; he could only watch as revenue continued to be encroached upon.
It's clear that the company doesn't want to repeat the same mistakes on the GLP-1 drug circuit. Along with rapid investment and production expansion, the company is also exploring more forms of administration, and next-generation products are ready to go.
A multi-pronged approach
Similar to Novo Nordisk, Eli Lilly's two syringe drugs are in serious shortage. Although the company is investing billions of dollars to expand production, the lengthy process still makes the “acute child” Rex anxious. He said, “We are building everything as soon as possible. The bad news is that it will take three to four years to build a new plant, ensure safe operation, prove the effectiveness of production... it will take a long time to scale up.”
One immediate solution is to make GLP-1 drugs in tablet form. Eli Lilly is developing an oral GLP-1 drug called orforglipron. According to Rex, unlike injections, production of oral medicines will expand much faster. There are many ready-made production systems in the world, and the company can quickly find partners to purchase production capacity.
The problem is that in order to make large, complex molecules such as tiverpotide into tablets, some other pharmaceutical companies have also failed in this process. According to normal progress, oral telpopeptide drugs will also have to go through the entire clinical trial process before they can be marketed. I'm afraid this is also a yearly wait.
Rex also revealed that the company currently has high hopes for a drug called retatrutide (retatrutide), a triple agonist drug because it simulates three appetite-suppressing hormones.
Eli Lilly revealed that retaglutide has a strong effect on visceral fat, fatty liver disease, and other types of obesity that are really difficult to treat. As a result, the weight loss effect after medication is even stronger than tiverpotide.
Retaglutide has now entered phase III testing, but it may take “several years” until it is sent to the FDA for review and marketing. Once stronger diet pills are on the market, it is expected that competition in the existing industry will increase. In addition to Novo Nordisk, large pharmaceutical companies such as Pfizer, Amgen, and Roche have also rushed into this racetrack with great momentum.
Rex said that Eli Lilly also has about “half a dozen” other weight loss drug programs. Some of them may achieve results, while others will fail. But he also stressed that only when we have multiple ways to fight the disease will we really see the disease become manageable and widely addressed.
