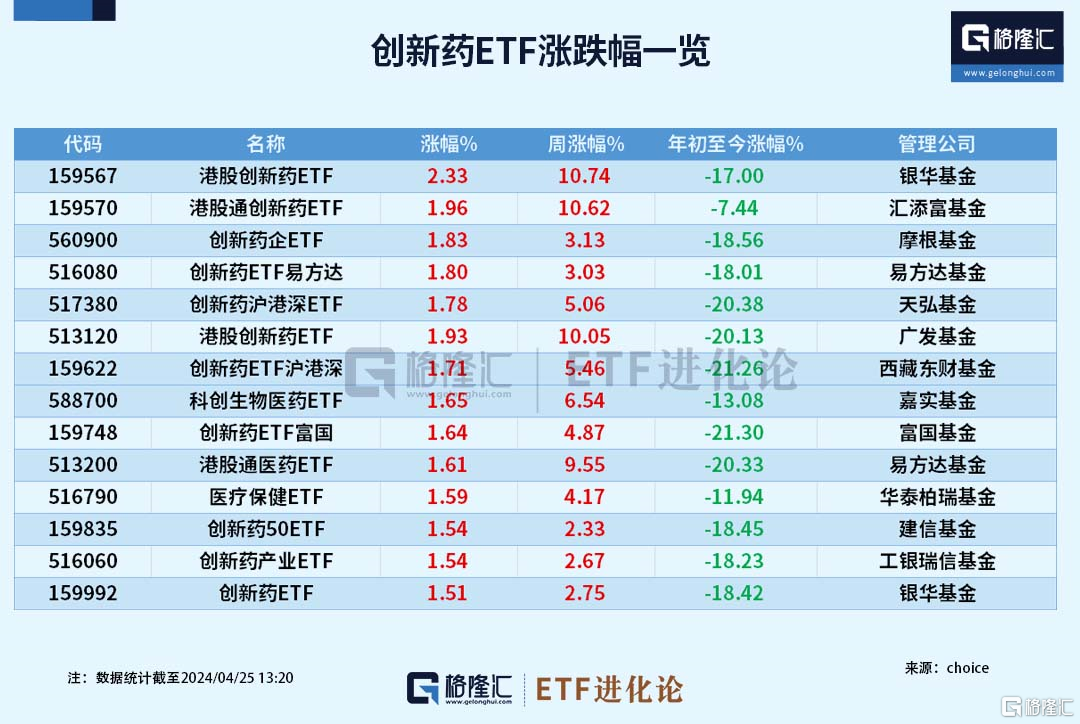
Innovative drugs rose. As of 13:20, Yinhua Fund's Hong Kong Stock Innovative Drug ETF rose more than 2.3%. Huitianfu Fund Hong Kong Stock Connect Innovative Drug ETF, E-Fangda Fund Innovative Drug ETF E-Fangda, Tianhong Fund Innovative Drugs Shanghai-Hong Kong Shenzhen ETF, Guangfa Fund Hong Kong Stock Innovative Drug ETF, Tibet Dongcai Fund Innovative Drug ETF Shanghai, Hong Kong Shenzhen, and Harvest Fund Sci-Tech Biopharmaceutical ETF, Fuguo Fund Innovative Drug ETF Fuguo, E-Fangda Fund Hong Kong Stock Pharmaceutical ETF, Huatai Healthcare Fund ETFs followed suit.
The Hong Kong stock market continued to rise this week. Yinhua Fund's Hong Kong Stock Innovative Drug ETF, Huitianfu Fund's Hong Kong Stock Connect Innovative Drug ETF, and Guangzhou Development Fund's Hong Kong Stock Innovative Drug ETF increased by more than 10% per week.
 Pharmaceutical stocks have risen. According to news, the national insulin collection contract was renewed and opened on April 23. The current collection volume is about 60% of the country's total demand, and the overall price reduction is about 3.8%. The bid period is until the end of 2027. The rules for this collection were gentle. It stipulated that 92% of the products were selected from Class A, Class B, and Class C. Among them, Ganli Pharmaceutical, Hefei Tianmai, and Yifan Biotech rose across the board, while Tonghua Dongbao dropped moderately and all were selected in Class A. The current collection renewal price favors leading domestic companies. While the prices of some domestic companies have risen, their share has also increased to a certain extent. We are optimistic about leading domestic companies that are deeply involved in the insulin industry.
Pharmaceutical stocks have risen. According to news, the national insulin collection contract was renewed and opened on April 23. The current collection volume is about 60% of the country's total demand, and the overall price reduction is about 3.8%. The bid period is until the end of 2027. The rules for this collection were gentle. It stipulated that 92% of the products were selected from Class A, Class B, and Class C. Among them, Ganli Pharmaceutical, Hefei Tianmai, and Yifan Biotech rose across the board, while Tonghua Dongbao dropped moderately and all were selected in Class A. The current collection renewal price favors leading domestic companies. While the prices of some domestic companies have risen, their share has also increased to a certain extent. We are optimistic about leading domestic companies that are deeply involved in the insulin industry.
Furthermore, on April 11, the National Health Insurance Administration issued the “2023 Statistical Report on the Development of Medical Insurance Services”. According to the Deputy Director of the Financial Regulations and Regulations Department of the National Health Insurance Administration, the overall operation of the health insurance fund in 2023 was stable, the co-ordinated fund achieved a reasonable balance, and the scope of use of the fund was further expanded. The medical insurance drug catalogue was expanded, and the reform of medical insurance payment methods continued to advance, and innovative drugs welcomed full process support.
Ten billion fund managers Ge Lan, Li Xiaoxing, and Zhao Bei discussed their views on pharmaceuticals in a quarterly report:
Looking ahead to the second quarter, the China-Europe fund Gülen is still optimistic about the relevant pharmaceutical industry chain driven by innovation.
Overseas, although the pace of interest rate cuts in 2024 is still uncertain, the overall trend of relatively loose liquidity has been determined. At the domestic level, the policy continues to strengthen support for innovation in the field of medicine and biology. As an important part of new quality productivity, pharmaceutical innovation is also the most determined direction of enterprise layout.
The number of innovative chemical drugs, biopharmaceuticals, and traditional Chinese medicine applications for marketing will continue to increase in 2023, and 2024 is expected to usher in the peak period of approval for marketing of related innovative drugs. Of course, we have also seen that the development of the domestic innovative drug industry chain is facing certain twists and turns and complications.
The healthy and sustainable development of the industrial chain requires unified coordination from various parties, such as corporate financing, product establishment, R&D promotion, registration applications, entry into the hospital procurement catalogue, compliant sales, and medical insurance payments. In response to the pain points of various links in the industrial chain, in recent years, the national level has also continuously carried out scientific coordination and precise clearance through policies and measures. The overall guiding direction of the industrial chain is still true innovation, mainly reflected in new targets, new mechanisms, new structures, drugs with independent intellectual property rights, and effective treatments to address clinical needs.
Predictably, support and guidance at the national policy level is expected to drive a new round of healthier and more sustainable R&D cycles, which also contain opportunities worth planning ahead of schedule. Furthermore, we are also actively watching the competitive medical industry chain's progress overseas. As the level of internationalization in the medical device field continues to advance, some companies have gradually achieved international breakthroughs in high-value chains and occupied overseas market opportunities, mainly in the fields of innovative equipment and in vitro diagnosis. Although the internationalization process of some enterprises will be affected by phased changes in the global geopolitical situation, the long-term core competitiveness of high-quality assets in the domestic innovation industry chain has not changed.
Yinhua Li Xiaoxing believes that due to short-term industry reasons and the high performance base for the first half of last year, the sector's performance in the next 1-2 quarters will not be very impressive, and there is some pressure on fundamentals. However, considering that the stock price has reflected most of the downside, we will also pay close attention to the possibility of improving the fundamentals of this sector and corresponding investment opportunities.
ICBC Zhao Bei pointed out that the innovative drug sector as a whole benefited from the end of the Federal Reserve's interest rate hike cycle. We believe that the R&D capabilities of domestic innovative drug companies are rapidly being integrated with the world, have gained global advantages in some sub-fields, and have been recognized by overseas multinational pharmaceutical companies. The “license-out boom” set off by the industry in the past 2 years is a concrete reflection of this industry trend. Against the backdrop of strict regulations, the domestic innovative drug industry has also ushered in “supply-side reforms.” This is beneficial to leading innovative drug companies with advanced product progress, strong R&D execution, strong capital reserves, and strong sales and fulfillment capabilities, because the degree of “internal volume” of the industry is expected to drop drastically in the future. Therefore, in the first quarter, we also increased the allocation of innovative pharmaceutical companies with strong R&D capabilities and potential for their products to go overseas. As for the innovative drug R&D outsourcing service (CXO) industry, although overseas demand for investment and financing and innovative drug research and development is expected to gradually pick up with the end of the Federal Reserve's interest rate hike cycle, the incident where leading Chinese CXO companies were suppressed by US lawmakers has increased uncertainty about the future development of the CXO sector, and we have also appropriately reduced geopolitical risk exposure in the investment portfolio. However, for CXO companies whose main source is domestic demand, although the current domestic new drug research and development boom has not improved significantly, it is also expected to gradually rise steadily in the future with active support from national policies.

 医药股上涨,消息面上,4月23日全国胰岛素集采续约开标,本次集采报量约为全国总需求量的60%,整体降价约3.8%,标期到2027年底。本次集采规则温和,规定了保A类、B类、C类中选价,92%的产品均中选,其中甘李药业、合肥天麦和亿帆生物全线上涨,通化东宝降幅温和且均为A类中选。本次集采续约价格利好国产龙头企业,部分国产企业价格上涨的同时,份额亦有一定提升,看好深耕胰岛素行业的国产龙头企业。
医药股上涨,消息面上,4月23日全国胰岛素集采续约开标,本次集采报量约为全国总需求量的60%,整体降价约3.8%,标期到2027年底。本次集采规则温和,规定了保A类、B类、C类中选价,92%的产品均中选,其中甘李药业、合肥天麦和亿帆生物全线上涨,通化东宝降幅温和且均为A类中选。本次集采续约价格利好国产龙头企业,部分国产企业价格上涨的同时,份额亦有一定提升,看好深耕胰岛素行业的国产龙头企业。