① Hebo Pharmaceuticals achieved revenue of US$89.502 million, a year-on-year increase of 119.9%, a total profit of US$22.763 million for the year, and a loss of US$137 million in the same period last year. ② Wang Jinsong revealed, “Of the revenue for the full year of 2023, close to 60 million US dollars came from Nona Biotech.”
“Science and Technology Innovation Board Daily”, March 29 (Reporter Zheng Bingxun) Three years after landing on the Hong Kong Stock Exchange, Hebo Pharmaceutical (2142.HK) turned a loss into a profit in annual finance for the first time.
In 2023, Hebo Pharmaceutical achieved revenue of US$89.502 million, an increase of 119.9% over the previous year, with a total profit of US$22.763 million and a loss of US$137 million in the same period last year. According to Heplatinum Pharmaceuticals, the growth in 2023 was mainly due to licensing partnerships with Pfizer, Cullinan Oncology, and Colombott.
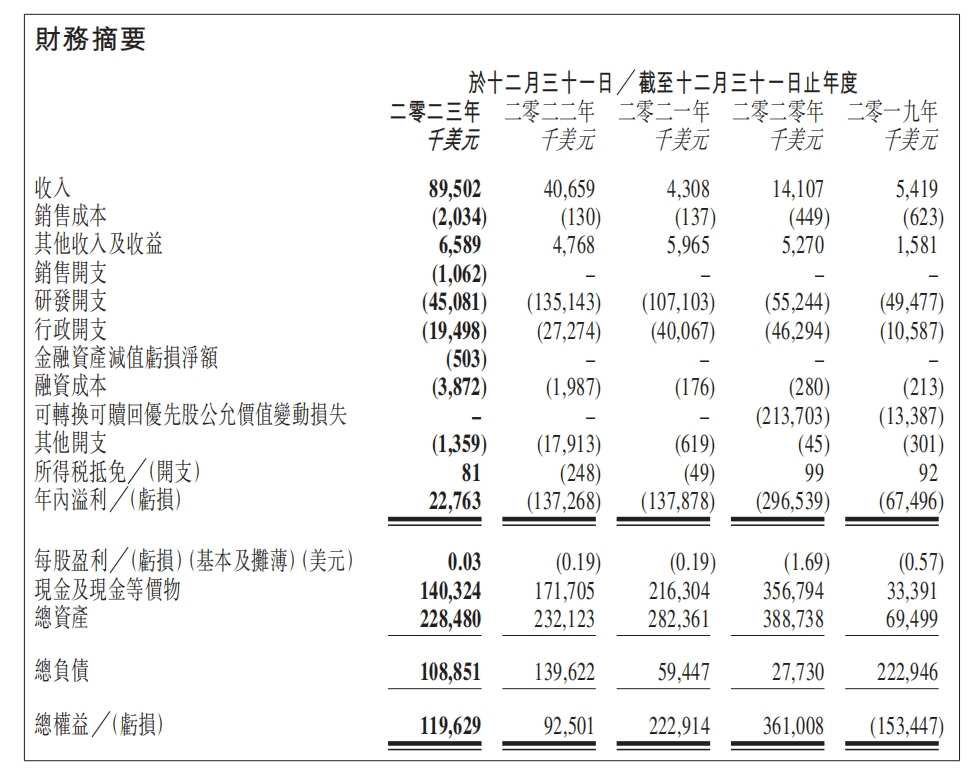
According to financial reports, in February and December 2023, Heplatinum Pharmaceuticals signed licensing agreements with Cullinan Oncology and Pfizer for its two products, HBM7008 and HNM9033, respectively. The former can bring Heplatinum Pharmaceuticals an advance payment of 25 million US dollars and a milestone payment of up to 600 million US dollars, while the latter can bring Heplatinum Pharmaceuticals an advance payment of 53 million US dollars and a milestone payment of up to 1.05 billion US dollars.
However, since there are still no commercialized marketed products, most of the revenue at this stage comes from external licensing cooperation, and the future continued profitability of Heplatinum Pharmaceuticals has attracted attention from the outside world.
In response to this situation, Wang Jinsong, founder, chairman and CEO of Hebo Pharmaceuticals, said at the performance briefing, “External licensing is actually the norm in the entire Biotech industry. Especially in the new environment, different companies will also have unique and leading characteristics in their own business. And Platinum Pharmaceuticals has both technical platform licensing and product licensing.”
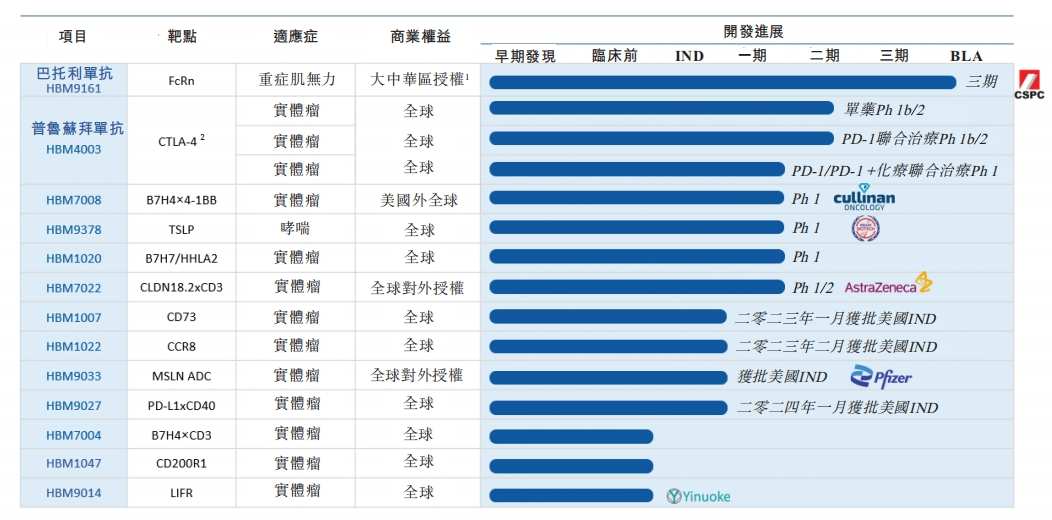
In the industry, Heplatinum Pharmaceuticals is seen as an example of successful transformation under the cold winter of capital. In October 2022, Hebo Pharmaceutical authorized bartolizumab (HBM9161), which is in the advanced clinical stage, to the Shiyao Group, completed clinical phase III of some projects, and established two sub-brands “Hebo Healthcare” and “Nona Biotech”, so that the two have a clear division of labor in different businesses.
Among them, Hebo Medical will focus on pipeline development, product cooperation and commercialization, while Nona Biotech will focus on providing global partners with overall “Idea to IND” (Idea to IND) solutions. In Wang Jinsong's words, “One focuses on product innovation, and the other focuses on technological innovation.”
Looking at the present, the revenue contributed by Nona Biotech, which focuses on technological innovation, is significantly higher than Hebo Healthcare, which focuses on product development. Wang Jinsong revealed at the performance conference, “Of the revenue for the full year of 2023, close to 60 million US dollars will come from Nona Biotech.”
Does this mean that future Heplatinum Pharmaceuticals will focus their development on Nona Biotech?
Wang Jinsong said that at present, the two divisions of the company have achieved independent operation, but they can mutually support the development of the group. The product line will be used as a breakthrough point, and the technology platform will be used as a supporting surface. The two complement each other to promote the further improvement of Heplatinum Pharmaceuticals business.
“The core clinical project is the backbone of the company. We will concentrate our resources to fully advance product research and finally commercialize it. At the same time, NanoBio will also further expand application scenarios in cutting-edge innovation fields, including technologies such as ADC, peptide coupling, nuclide coupling, artificial intelligence, next-generation cell therapy, and mRNA, making it an even more scarce strategic resource and attracting more high-quality partners.” Wang Jinsong said.
It is worth mentioning that since the Phase III clinical trial of Bartolizumab for myasthenia gravis was postponed in December 2023, there has been no recent progress. Heplatinum Pharmaceuticals said that in December of last year, the voluntary plan included other long-term safety data and re-submitted the BLA for bartolizumab. It is expected that the BLA will be re-submitted to the NMPA in the first half of 2024.
In response to this incident, Wang Jinsong did not disclose any further information. He only stated that the new application is progressing according to the original plan, and that the commercialization side is also closely cooperating with CSPC Group. “According to the new drug declaration schedule, we will further refine the commercialization arrangements.”
By the end of 2023, Hebo Pharmaceutical had more than 10 drug candidates for cancer and immune diseases. Among them, bartolizumab is the fastest progressing drug of all drugs and is most likely to be the first to bring in product revenue to Hebo Pharmaceutical. The product was introduced by Hebo Pharmaceutical from HandAll in 2017 and authorized to CSPC Group in December 2022.