① The cooperation was not negotiated. Belcher filed a lawsuit against Cansino, claiming loss of earnings and moral damages totaling about RMB 241 million; ② Cansino claimed that the lack of cooperation was due to the failure to register the COVID-19 vaccine locally in Brazil, and due diligence discovered that Belcher had some negative rumors.
“Science and Technology Innovation Board Daily”, March 14 (Reporter Zheng Bingxun) While performance experienced a sharp decline last year, Cansino (688185.SH, 6185.HK) was also involved in a lawsuit with Brazilian company Belcher. It can be described as if the house leak happened overnight.
Cansino revealed that as early as 2021, in order to submit an application for emergency use of the recombinant novel coronavirus vaccine (adenovirus type 5 vector) to Brazil's National Health Surveillance Agency (Anvisa), a “Letter of Authorization” was issued to the local supplier Belcher in April of the same year, authorizing Belcher to negotiate with local government agencies on the registration and commercialization of the COVID-19 vaccine in Brazil.
However, due to the fact that the important conditions for cooperation with Belcher were not met in a timely manner, Cansino sent a “Notice of Withdrawal of Authorization Letter” to Belcher almost 2 months later, which clearly took effect immediately on the day it was issued.
However, almost 2 years later, Belcher suddenly filed a lawsuit against Cansino, demanding that Cansino pay a total of about 167 million reais (about 241 million yuan) for “loss of earnings” and “moral damages.”
As an investor, the “Science and Technology Innovation Board Daily” reporter learned from Cansino's board of directors that the above important cooperation conditions were not met, which meant that the COVID-19 vaccine had not been registered locally in Brazil. This was a prerequisite for licensing the product to Belcher, so Cansino initiated the cancellation against the other party. On the other hand, when the company applied due diligence against Belcher in the early stages, it was discovered that the other party had some negative rumors in the local area. “Therefore, the revocation of the authorization was the result of our considerations based on multiple considerations.”
In fact, prior to entering Brazil, Cansino's recombinant COVID-19 vaccine (adenovirus type 5 vector, including inhalation dosage forms) began in early 2022 and was approved for use in many places at home and abroad.
Among them, in May 2022, the WHO (WHO) added Cansino's recombinant COVID-19 vaccine to the “emergency use list”. In September of the same year, the inhaled dosage form of the product was included in emergency use by the National Drug Administration as a booster. Following that, Morocco and Indonesia also included the product for emergency use, respectively.
As to why it was not possible to obtain registration in Brazil, the director and secretary mentioned above said that it is a complicated process to obtain approval for emergency use locally. On the one hand, it is necessary to consider not only the product's entry conditions in the local area, but also the considerations of public health regulators.
According to information, if a foreign vaccine company wants to apply for emergency use authorization in Brazil, it is necessary for Brazil's Anvisa to conduct a contract review with the authorized representative of the foreign vaccine company in the region. This is a common practice in Brazil.
According to overseas media reports, as early as the evening of June 28, 2021, local time, Anvisa claimed to have received an email from Cansino regarding the cancellation of the authorized cooperation with Belcher. After voting, the Anvisa Council decided to cancel the application for emergency use of the Cansino vaccine in Brazil. According to the initial cooperation intention, Brazil's Ministry of Health plans to purchase 60 million doses of the Cansino vaccine, priced at $17 per dose.
The reporter further asked Cansino's secretary. What is the specific basis for Belcher's claim of 241 million yuan? The other party said, “This is Belcher's calculation based on some paperwork provided by itself, but this amount will definitely change as the lawsuit progresses.”
Furthermore, regarding Belcher's claim for moral damages, the other party said it was also surprised. “It is reasonable to say that the plaintiff is a company, but it has submitted compensation for moral damage. The company is actively discussing this claim with a professional legal team.”
Therefore, it can also be seen that Cansino clearly stated in the announcement that it has a strong defensive position. “The main reason is that we have not signed any other written agreement with the other party other than signing a “Letter of Authorization” with the other party and later legally revoking it.” A member of the Board of Directors and Secretaries added.
In fact, this isn't the first time Cansino has been passive when it comes to vaccines. In February of this year, based on the current decline in market demand, Cansino announced that the subsidiary “Shangyao Cansino”, which produces recombinant COVID-19 vaccines, would be excluded from the scope of the consolidated report. Prior to that, news of the discontinuation of production of Shangyao Cansino had already spread.
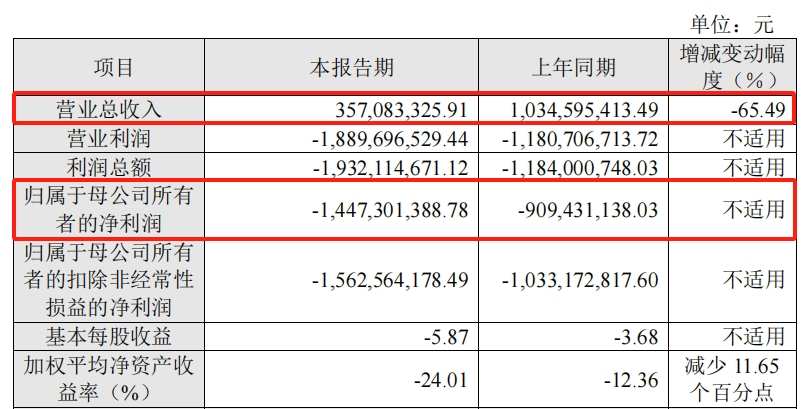
In 2023, Shangyao Kangxino achieved revenue of 339.46,800 yuan, a year-on-year decrease of 78.49%, and a net loss of 956 million yuan. Affected by this, Cansino achieved revenue of 357 million yuan in 2023, a year-on-year decrease of 65.49%, a net loss of 1,447 million yuan to mother, and a loss of 909 million yuan in the same period last year.