① Olin Biotech initially confirmed that the marketing application for its AC-Hib combination vaccine was not approved. Previously, it was regarded as a “new performance growth point.” ② Olin Biotech is facing declining performance and declining product gross margin year by year.
“Science and Technology Innovation Board Daily”, January 31 (Reporter Zheng Bingxun) “If (the product) does not appear in the approval section, it can be understood that it has not passed the approval.” Today, the “Science and Technology Innovation Board Daily” reporter obtained initial confirmation from Olin Biotech (688319.SH) securities department personnel as an investor. The marketing application for the AC-Hib combination vaccine developed by Olin Biotech was not approved, confirming the rumor.
Affected by this, when A-shares opened today, Olin Biotech's stock price turned green. At one point, it fell nearly 7 percentage points during the intraday period. By the close of the day, the stock price had fallen 5.83% to 10.83 yuan/share.
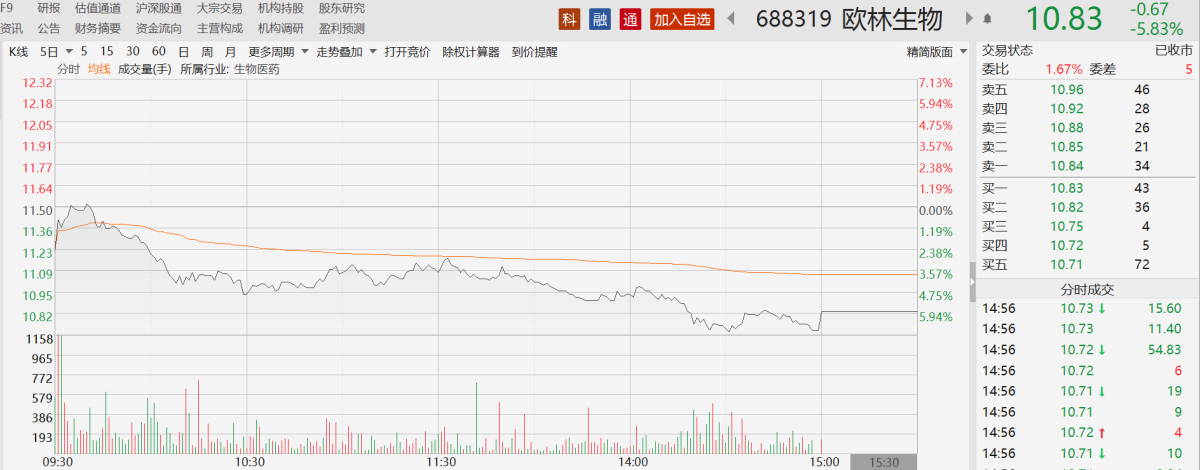
▌“ Fallen” performance growth points
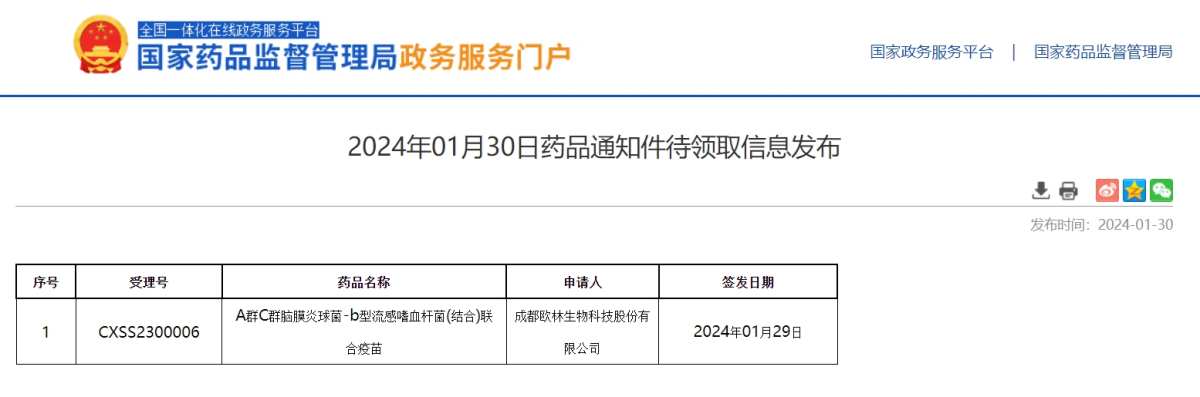
The official website of the National Drug Administration (NMPA) also shows that Olin Biotech's “Group A Group C Meningococcus - Haemophilus influenzae type b (conjugate) vaccine” appeared under the “Drug Notice to be Received” section rather than the “Drug Approval Information” section.
According to reports, according to convention, only drugs that have not been approved will appear in the first method, but specific reasons may include refusal of approval and voluntary withdrawal by the company.
However, judging from public information, Olin Biotech has not announced the withdrawal of the marketing application for the AC-Hib combination vaccine. However, the company personnel mentioned above said, “The company has yet to receive the final approval from the Drug Administration. We haven't seen the specific reason yet; it will be announced as soon as it is received.”
“Regarding this vaccine, there are definitely further measures to be taken, but they also need to be judged based on the current situation.” The other party further added.
Olin Biotech's AC-Hib combination vaccine was first accepted by the NMPA in February 2023. Since there are no similar products on the market, Olin Biotech has high expectations for its launch, and bluntly stated that it is a “new performance growth point.”
According to data, the AC-Hib combination vaccine is suitable for children aged 2 months to 5 years and can simultaneously prevent infectious diseases such as meningitis, pneumonia, sepsis, cellulitis, arthritis, and epiglottitis caused by group A, group C meningococci, and Haemophilus influenzae type b.
Compared to single vaccination, Olin Biotech's AC-Hib vaccine can reduce the number of vaccinations, prevent multiple diseases at the same time, and is more compliant. Furthermore, since it uses a freeze-dried dosage form, it is easy to store and transport, and the safety is higher.
As of the first half of 2023, Olin Biotech has invested 476.988 million yuan in R&D of the AC-Hib combination vaccine, which has exceeded the estimated investment by 11.84%.
It is worth mentioning that Olin Biotech is also facing a potential competitor — Zhifei Biotech (300122.SZ) in the development of the AC-HiB joint vaccine.
Zhifei Biotech announced that it will step up the development of a freeze-dried form of the AC-Hib combination vaccine, or similar product of Olin Biotech, after being rejected in 2020 due to the remarketing application of its AC-Hib combination vaccine (injectable dosage form). In the first half of 2023, Zhifei Biotech's R&D investment for this new vaccine increased by 139,500 yuan, with a total investment of 78.0318 million yuan.
▌Another pole of growth?
The reason why Olin Biotech has high expectations for the launch of the AC-Hib combination vaccine is closely related to its performance, which is under pressure year by year.
According to the data, in 2022, Olin Biotech achieved revenue of 547 million yuan, a year-on-year increase of 12.38%, and net profit of 265.771 million yuan, a year-on-year decrease of 75.38%. In the first three quarters of 2023, revenue was 352 million yuan, a year-on-year decrease of 4.98%, and net profit to mother was 30.2838 million yuan, a year-on-year decrease of 24.62%.
Up to now, Olin Biotech has a total of 3 marketed vaccine products, namely: adsorbed tetanus vaccine, Hib conjugate vaccine, and AC conjugate vaccine. In the face of rising product revenue, gross margins declined year by year, to 95.14%, 93.93%, and 92.86% respectively in 2020-2022.
The “Science and Technology Innovation Board Daily” reporter noticed that in Olin Biotech's research project, in addition to the AC-Hib combination vaccine, there is also a “recombinant Staphylococcus aureus vaccine” in phase III clinical trials. All other products are in the early stages of preclinical research. This means that the recombinant Golden Grape vaccine may replace the AC-Hib combination vaccine and become Olin Biotech's new expectation for a performance growth point.
According to information, the recombinant golden grape vaccine will be used to prevent Staphylococcus aureus infection after surgery. It is a class 1 biological product for prevention. There are no similar products on the market at home or abroad.
Regarding this vaccine, Olin Biotech Securities Department personnel revealed, “Phase III clinical trials will begin in 2022. Because 6,000 cases were enrolled and the sample size is quite large, the clinical trial may take 2 or more years to complete. An overdue analysis was carried out at the end of last year, and it was recommended that the company continue to advance the research. This is also in line with the company's expectations.”
According to the data, as of the first half of 2023, the total R&D investment for the recombinant golden grape vaccine was 204 million yuan, and the total R&D investment is expected to reach 630 million yuan.
