① Shortly after Jushi Biotech authorized the ADC drug SYS6002 to Corbus, New Novell increased its capital by 1,881 billion yuan, bought 51% of the other party's shares and became the controlling shareholder. ② Recently, Sinovay once again announced that it will acquire 100% of the shares of Shiyao Baike by issuing shares and cash, and that Shiyao Baike is developing a layout in the GLP-1 field.
“Science and Technology Innovation Board Daily”, January 29 (Reporter Zheng Bingxun) Phase I clinical data for an ADC drug led the stock price of US partner Corbus (NASDAQ: CRBP) to skyrocket 149% in one day. As a result, the capital market is looking forward to New Novus (300765.SZ).
The origin of the incident is that the ADC drug mentioned above is SYS6002, an oncology product developed by Jushi Biotech. In early 2023, Jushi Biotech granted Corbus its commercial rights in the US, Europe, the UK, etc., and received a down payment of 7.5 million US dollars, and is expected to receive an additional 685 million US dollars in potential cooperation funds.
However, in September of last year, New Novus “broke out”. In September of last year, it offered a capital increase of 1,881 billion yuan to buy 51% of the other party's shares in one go and become the controlling shareholder, and the other party was included in the scope of the consolidated statement. At the time, Jushi Biotech's net worth was around 689 million yuan.
This is a move that the new Novus has to take the initiative to seek change in the face of increased competition on the functional food circuit.
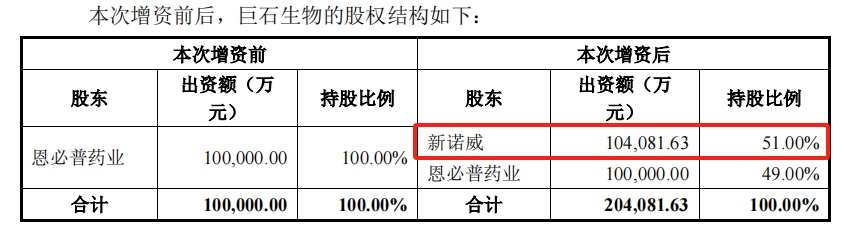
The deal was completed in early January of this year. Less than a month later, Corbus presented phase I clinical data for SYS6002 at the 2024 ASCO-GU conference, showing that the ORR (objective response rate) for people with mixed tumors was 43% and was well tolerated. There have been no discontinuation or dose reduction in the study so far.
On the way to change, Novus seems to have unearthed a “treasure.”
However, judging from actual performance, investors don't seem to have long-term confidence. When A-shares opened on Monday, the share price of New Novus fell 1.6 percentage points, and the intraday decline continued to decline. By the close of the day, Sinovay's stock price had fallen by 14.08% to 28.07 yuan/share.
It must be pointed out that SYS6002 is currently only undergoing phase I clinical trials, and there is still a long way to go before it is actually commercialized. However, the future direction of the new Novi, which is speeding up the layout of a new circuit, is also unknown. The “Science and Technology Innovation Board Daily” reporter sent a letter to Xinover to inquire about future development directions, etc. As of press time, no response had been received.
▌Increase capital of Megalithic Biology and enter the field of oncology
Behind the huge capital increase, Megalithic Biotech shows a new Novus that is eager to break through.
Novus is an indirect shareholding company of Shijiyao Group (1093.HK), focusing on the functional food business in the health field. Its main products include functional ingredients such as caffeine, acarbose, and anhydrous glucose, as well as some health foods, such as vitamin C tablets.
With the addition of more peer companies, competition on the functional food circuit is intensifying, and New Novi faces pressure from slowing performance growth and declining gross margin year by year.
In the first three quarters of 2023, Novus achieved revenue of 1.902 billion yuan, a slight increase of 0.46% year on year; net profit to mother was 583 million yuan, an increase of 12.74% year on year. In contrast, revenue and net profit growth rates for the first three quarters of 2022 were 47.42% and 89.25%, respectively.
Between 2020 and 2022, the gross sales margin of Novus's functional food products declined year by year, corresponding to 55.66%, 47.90%, and 43.21%, respectively. In the first half of 2023, the gross margin of functional ingredients increased slightly by 3.82% year-on-year, while the gross margin of health food products decreased by 3.74%.
The significance of Neo Novi's capital increase in Destone Biotech is not only to become the latter's controlling shareholder, but also to enter a new circuit. In terms of business, Jushi Biotech focuses on cutting-edge biopharmaceutical fields such as antibody drugs, antibody-conjugated drugs (ADC), and mRNA vaccines. An mRNA vaccine was included in emergency use in China at the end of last year. Dushi Biotech has more than 20 research projects. The main treatment fields include breast cancer, cervical cancer, gastric cancer, psoriasis, etc. Among them, 3 products are in clinical phase II/III.
The ADC drug SYS6002 mentioned above is only one of Jushi Biotech's overseas products. The drug targets Nectin-4. This target has high expression levels in various cancers such as bladder cancer, lung cancer, and breast cancer, and has become a potential target for treating various cancers.
Prior to SYS6002, Jushi Biotech's other ADC drug, Sysa1801, also reached a cooperation with Elevation Oncology. In addition to receiving a down payment of 27 million US dollars, Dushi Biotech is also expected to receive up to 1,168 million US dollars in potential cooperation payments. Both SYSA1801 and SYS6002 are currently in Phase I clinical trials.
As of January-July 2023, Jushi Biotech achieved revenue of 347.010,000 yuan, R&D investment of 341 million yuan, net loss of 335 million yuan, and cash flow of 517 million yuan.
▌If you want to buy 100 grams of petrochemical, then seek GLP-1
On the road to transformation, Novus also has greater ambitions.
Just two days before Corbus released clinical data on SYS6002, Sinovay once again announced that it would acquire 100% of the shares of Shiyao Baike by issuing shares and cash. Currently, Shiyao Baike is wholly owned by the Shiyao Group indirectly. Purchase consideration has yet to be determined, and Novo is expected to disclose it in its annual report.
New Novi will issue shares to direct shareholders of Shiyao Baike at a price of not less than 20.91 yuan/share, and the cash purchase portion will not exceed 10%. At the same time, it plans to issue shares to no more than 35 specific investors to raise capital.
Currently, Shiyao Baike focuses on cutting-edge fields such as long-term protein drugs. Its core product “Jin Youli” is the first self-developed long-term whitening drug in China. It can prevent infections and fever caused by neutropenia in chemotherapy patients. This product is also one of the pillar products of the CSPC Group.
According to BOC International, sample hospital data for September 2023 showed that Jin Youli's market share reached 38%. In 2023, Shiyao Baike achieved revenue of 2,663 billion yuan, net profit of 859 million yuan, total assets of 4.719 billion yuan, and total liabilities of 635 million yuan.
In addition to long-acting whitening formulations, Shiyao Baike is also developing a layout in the GLP-1 field. The main products under development include TG103 and Class 2.2 new drug simeglutide (chemical synthesis method), pre-clinical long-acting simeglutide (fluid crystal dosage form), oral simeglutide, and GLP-1 dual-target/triple-target drug candidates.
Shiyao Baike expects that TG103 and chemically synthesized simeglutide will submit a marketing application in 2025 and are expected to be officially launched in 2026. Among them, the TG103 weight loss phase III trial has already been enrolled, and stage III diabetes will also be enrolled in the group soon.
According to reports, GLP-1 receptor agonists have been widely recognized in the international market. They have surpassed insulin to become the most widely used treatment for type 2 diabetes in the world in 2023.
According to CIC Insight and Consultation, the GLP-1 receptor agonist market for type 2 diabetes in China has expanded from 700 million yuan in 2018 to 6 billion yuan in 2022, and is expected to grow further to 66.7 billion yuan in 2032. The market size of GLP-1 receptor agonists for obesity and overweight in China will increase from 400 million yuan in 2023 to 45.5 billion yuan in 2032.
Meanwhile, global sales of simeglutide products reached 10.9 billion US dollars in 2022, making it one of the top ten best-selling drugs in the world in 2022, and is likely to become the top three best-selling drugs in the world in 2023.
