
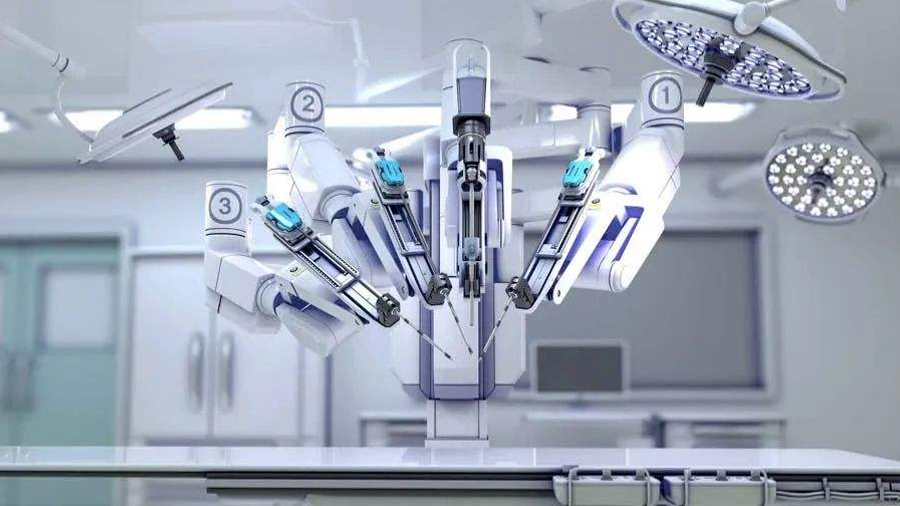
 The first company in China to have completed key clinical trials of porous and single-hole endoscopic surgery robots.
The first company in China to have completed key clinical trials of porous and single-hole endoscopic surgery robots.
This article is for IPO to know the original.Author | RobinOfficial account of Wechat | ipozaozhidaoAccording to IPO, Shenzhen Jingfeng Medical Technology Co., Ltd. (hereinafter referred to as "Jingfeng Medical") updated its prospectus a few days ago to list on the main board, with Morgan Stanley, China International Capital Corporation and Citi as co-sponsors.
Founded in 2017, Jingfeng Medical is committed to the design, development and manufacture of surgical robots. The pipeline now covers porous and single-hole endoscopic surgery robots, natural cavity surgery robots and traditional minimally invasive surgical instrument products. Help to provide comprehensive minimally invasive surgical solutions to complex problems. Except for robot ultrasonic scalpel, robot stapler, stereoscopic endoscope and intelligent stapler are classified as category II medical devices, all Jingfeng medical products under research are classified as category III medical devices.
Among them, the core product of Jingfeng Medical is Jingfeng porous endoscopic surgery robot MP1000, and a key research product single-hole endoscopic surgery robot SP1000--MP1000 and SP1000 are qualified to conduct rapid examination of innovative medical devices of the State Drug Administration through the green channel. MP1000 was registered by the State Drug Administration in December 2022 for use in urological surgery.
According toFrost SullivanThe report.Jingfeng Medical is the first company in China to have completed key clinical trials of porous and single-hole endoscopic surgery robots..
Specifically, the core product MP1000 is designed to help doctors perform a variety of minimally invasive operations, including urology, gynaecology, general surgery and thoracic surgery.The clinical data of the registration trial of MP1000 show that the clinical performance of MP1000 is excellent, and the effectiveness and safety shown in the head-to-head comparison is not lower than that of Leonardo da Vinci, the world's leading surgical robot..
SP1000, the key research product of Jingfeng Medical, is a supplement to MP1000. Jingfeng Medical started a registered clinical trial of SP1000 in gynecological surgery in October 2021. According to Frost SullivanSP1000 is the first single-hole surgery robot in China to enter a critical clinical trial of gynecological surgery, which has been basically completed and is expected to be completed in the first quarter of 2023. Single-hole endoscopic surgery robot represents the development trend of minimally invasive surgery, and has always been one of the research focuses of the world's leading surgical robot companies..
In addition to MP1000 and SP1000, Jingfeng Medical has five products under development, which expand the range of products from robot-assisted minimally invasive surgery to non-invasive natural cavity surgery robots and traditional minimally invasive surgical instruments. Among them, Jingfeng bronchoscope robot is used in natural cavity endoscopic surgery ("NOTES") surgery. Jingfeng Medical completed the design of the first generation principle prototype robot and began animal testing in January 2022. In addition, two types of traditional minimally invasive surgical instruments being developed by Jingfeng Medical include Jingfeng stereoscopic endoscope (approved by Guangdong Provincial Drug Administration in October 2022) and Jingfeng intelligent stapler.
Jingfeng Medical has an annual production capacity of about 80 surgical robots with a total area of about 8000 square meters.The annual production capacity of 500 surgical robots is planned to be built in Shenzhen this year, and it is expected to become the largest production base of surgical robots in China.. In terms of marketing, Jingfeng Medical cooperates with hospitals that have set up training centers to provide training and trial of surgical robots. Training centers have been set up in 10 cities, and it is planned to build 20 to 30 regional training centers in cooperation with top hospitals in the next two or three years, so that marketing activities will cover no less than 95% of the domestic third-class and first-class hospitals.
Since its establishmentJingfeng Medical treatmentCumulative completion of 6 rounds of financingThe total amount is 2.05 billion yuanInvestors include dozens of well-known institutions, including Sanzheng Health, LYFE Capital, Guangfa Xinde, Boyu Capital, National Policy Investment, Lenovo Star, Temasek, Poly Capital, Sequoia Capital China, Xiangfeng Investment, OrbiMed, Morning one Investment and so on..
Jingfeng Medical said in its prospectus that the net funds raised by IPO will be mainly used for the continuous research and development and future commercialization of the core product MP1000; for the continuous research and development of other pipeline products under development, as well as for future manufacturing and commercialization; for potential strategic acquisitions and cooperation in surgical robots and related fields; and for working capital and general corporate purposes.
This article was originally written by the official account IPO (ID:ipozaozhidao). If you need to reprint it, please contact Uncle C ↓↓↓.





Honey snow ice city| |Adopt a cow| |Mother and uncle| |Braised Chicken, Dezhou StyleMiss food.| |Rural base| |Delma.| |Home furnishings to EuropeKeep| |The fourth paradigm| |Yinming biology| |Hony investment

