Structural heart disease, as a common disease that seriously threatens the health of people, especially the middle-aged and elderly, has the characteristics of high prevalence rate, high disability rate and high mortality rate. At present, how to effectively treat structural heart disease has become an urgent clinical problem to be solved.
In this context, the interventional treatment of structural heart disease has become the focus of exploration and research direction of many pharmaceutical companies around the world, which may contain new investment opportunities.
Recently, Lepu Xintai Medical Technology (Shanghai) Co., Ltd. (hereinafter referred to as "Xintai Medical", stock code: 2291.HK), the leader in interventional treatment of structural heart disease, is conducting a public offering in Hong Kong. As a subsidiary of Le Pu Medical, which is the first to enter the field of cardiac interventional medical devices in China, Xintai Medical has become the most comprehensive interventional medical device provider covering structural heart disease in China.
It is reported that Xintai Medical IPO by China International Capital Corporation as its exclusive sponsor, former IPO investors including Weiwu Capital, Sequoia Capital, Shanghai Biopharmaceutical Industry Equity Investment Fund Partnership, CDH Capital shareholding, Huaihua Haozhi and other well-known investment institutions endorsement. The listing of Xintai Medical in Hong Kong provides us with a good observation window.
1. The technology + market is gradually mature, and it is the right time for structural heart disease to intervene.
Reviewing the development trend of the treatment of structural heart disease in China, in the past, structural heart disease was mainly treated by surgery, with great trauma and high risk of operation. Nowadays, interventional therapy has gradually begun to replace traditional surgical surgery with the advantages of simple operation, less pain, scars and complications, lower risk of infection, shorter hospital stay and recovery time.
Although from a technical point of view, compared with foreign countries, the interventional therapy of structural heart disease in China started relatively late, but in recent years, with the continuous emergence of new technologies and new instruments, the interventional therapy of structural heart disease marked by TAVR (transcatheter aortic valve replacement) tends to mature.
Based on their rich experience, many domestic interventional medical device manufacturers, such as minimally invasive Medical Care, Qiming Medical and Xintai Medical, have been able to gradually catch up with or even surpass foreign manufacturers in terms of technology, ideas and device innovation.It can be seen that the interventional therapy in the field of structural heart disease has been on the right track and is in the stage of rapid development.
In terms of market scale, with the continuous extension of life expectancy in China and the gradual intensification of the aging process of the population, the number of patients with structural heart disease is increasing. Therefore, the market scale of interventional medical devices for structural heart disease in China is growing rapidly.
According to Frost&Sullivan data, China's interventional medical device market for structural heart disease has increased from 400 million yuan in 2017 to 2 billion yuan in 2021, with a compound annual growth rate of 48.3 percent, and is expected to reach 10.4 billion yuan in 2025 and 51 percent from 2021 to 2025.
It is not difficult to see that China's interventional medical device market for structural heart disease has entered a period of accelerated volume and continues to open up room for growth.
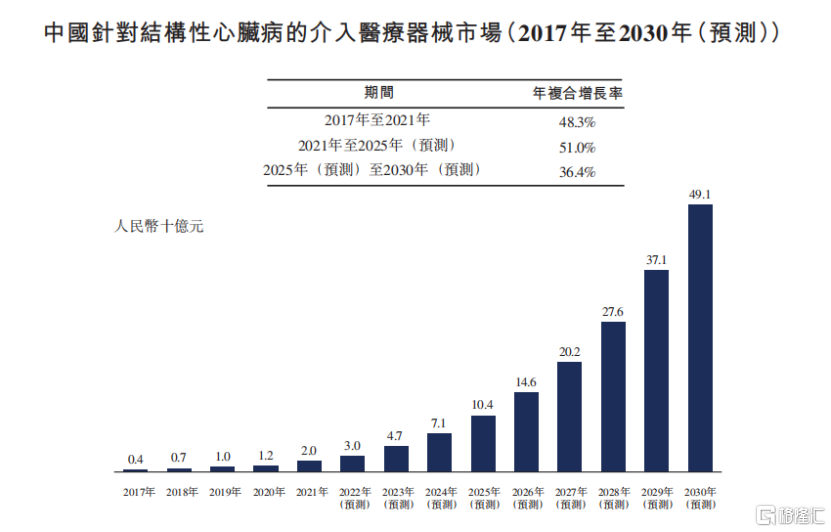
Source: prospectus
On the whole, whether it is the iterative upgrading of technology or the expansion of the market scale, the interventional treatment market of structural heart disease in China has gone beyond the groundwork of early time and capital investment and entered the door of preliminary maturity.
Back to the investment perspective, the author believes that in the future industry, a few domestic manufacturers will be able to grow into the main players in the market.Among them, domestic manufacturers with leading technological advantages, commercial products, first-mover advantage in the market and good profitability are expected to drive into the fast lane of development and stand out from the race track.
2. With the technical "hard power" as the support, the overall layout of the product pipeline blossoms at multiple points.
Drawing lessons from the development process of global medical device leaders, it can be seen that strengthening their own technical strength and expanding product lines in a critical period is an excellent means to maintain a market leading position and occupy market share for a long time.From this, it is not difficult to see that innovative technology and product layout is one of the capabilities to reflect the core competitiveness of medical device manufacturers.
As a pioneer in the field of interventional medical devices for structural heart disease in China, Xintai Medical has been deeply involved in the field of interventional medical devices for structural heart disease since its establishment in 1994. at present, it has become the most comprehensive interventional medical device provider covering structural heart disease in China.
In my opinion, Xintai Medical can develop to such a leading position in the industry, which is inseparable from its leading technological advantages. It is reported that the company takes the R & D concept of "intervention without implantation, implantation without residue" as its iterative direction of structural technological innovation, and has independently developed three innovative technologies including biodegradable technology, ultrasonic technology and radio frequency puncture technology.
It is worth mentioning that the Frost&Sullivan data showsXintai Medical was completed in 2018.The first complete biodegradable VSD interventional therapy in the worldIs a pioneer in the application of degradable technology to the field of structural heart diseaseMarks the global field of completely biodegradable occluders.Achieved great significance.Break through。
At the same time, Xintai Medical also has a large number of domestic and global innovation patents to build a technical advantage moat for Xintai Medical in the field of interventional therapy. So far, the company has 232 registered patents and 51 pending patent applications in China, as well as 14 pending patents in the United States and the European Union, which has also laid a solid foundation for its future international expansion.
Based on the leading technological advantages, Xintai Medical Co., Ltd. conforms to the development trend of the industry and the pain points of the market demand, extensive and comprehensive layout of the interventional medical device market for structural heart disease mainly includes three major application areas (congenital heart disease, cardiogenic stroke and valvular disease).
Subdivided, Xintai Medical's product portfolio can be roughly divided into two categories: occluder products and heart valve products. at present, the company has successfully developed 20 listed occluder products and 9 occluder products under development. and 21 major heart valve products, the comprehensive product layout also shows a strong anti-risk ability.
In the field of occluder products, Xintai Medical continues to promote the upgrading of its products in the field of congenital heart disease and cardiogenic stroke through the application of innovative technology. In February this yearAs the world's first degradable occluder, the company'sMemoSorb ®Fully degradable occluder systemHas been approved by the State Drug Administration.Listing leads the interventional treatment of structural heart disease from the era of metal occlusion to the era of degradability.. At the same time, we are also concerned about the company's application of biodegradable technology to a number of product research and development, the company's biodegradable PFO occluder has completed clinical selection and follow-up, will be reported to the State Drug Administration for registration; degradable atrial septal occluder has also completed clinical selection into the follow-up phase, is expected to start registration in 2023; biodegradable left atrial appendage occluder has completed type test and animal experiments, and is expected to enter the clinical stage in the fourth quarter.


Source: prospectus
In the field of heart valve products, Xintai Medical has developed a comprehensive portfolio of heart valve products under development, covering all major valvular diseases, including aortic valve, mitral valve and tricuspid valve.
Among them, the transcatheter implantable aortic valve system being developed by the company is expected to be 100% deployable, recyclable and repositioned before being separated from the delivery system, showing obvious innovative features. At present, all commercial transcatheter implantable aortic valve systems in China do not have this characteristic.
It is worth noting that at present, the permeability of heart valve products in China is still low, and the volume has not been achieved. The author believes that with the investment of time and funds, the market of heart valve products in China may usher in explosive growth from 2024 to 2025.
Xintai Medical expects its first commercial heart valve product to be launched in 2025, according to the prospectus. The author believes that if the clinical progress of heart valve products progresses smoothly, it is expected to bring new performance growth points for the company and achieve curve overtaking.


Source: prospectus
3. Strong ability of self-hematopoiesis and continuous release of intrinsic value
While having the leading technology and continuous iterative upgrading of the product portfolio, investors are also more focused on fundamentals when making the target choice. It pays more attention and attention to whether the company can realize its products quickly and form a sustainable self-hematopoietic ability.
Xintai Medical adopts the way of "open source" and "cost saving" as its core strategy for commercialization, so as to achieve the goal of sustained growth in performance.
On the one hand,As a subsidiary of Lepu Medical, Xintai Medical, with its strong market and marketing resources in the medical device market, can quickly achieve commercialization and bring revenue growth after the iteration of innovative products.
According to the prospectus, the company's revenue increased from about 117 million yuan in 2019 to 223 million yuan in 2021, with a compound annual growth rate of 38.6 percent.It is a rare profitable and fast-growing enterprise in the field of interventional treatment of structural heart disease in the Hong Kong stock market.. At the same time, according to the revenue recognized by Chinese sales in 2021, Xintai Healthcare accounts for 38% of the market share, making it the largest manufacturer of congenital heart disease occluder products and related surgical products.

Source: prospectus
On the other handOn the basis of ensuring that sales continue to increase, Xintai Medical also effectively controls the cost of sales. In recent years, the proportion of sales cost to revenue in the same period has been kept at a stable and low level, which can effectively improve the company's profitability.

Source: prospectus
From this, it can be seen that Xintai Medical's business model and profitability have been well verified, which also reflects the certainty of steady growth after listing in the volatile market in the future.
4. Conclusion
Returning to the capital market, the medical device sector is now in a state of "valuation bottom + allocation low + emotional freezing point". As a series of policies, such as the exclusion of innovative medical devices from the scope of collection, the landing of the spine, and financial discount loans to boost new medical infrastructure, continue to release positive signals, the market's concerns about the policy have been relieved to a certain extent, and the medical device sector is expected to usher in a strong performance. On the other hand, Xintai Medical relies on the leading technology platform to continuously promote the iteration of product update, which makes the company have the advantage of innovation and reduce the risk in the face of collection.
With the official launch of Xintai Medical IPO, with the company's strong comprehensive strength and market first-mover advantages, coupled with its parent company Lepu Medical in terms of business synergy. The author believes that it is expected to stand out from the new round of medical device market under the blessing of capital.
