Edited by Soochow Securities, "the first domestic PD-1 has been approved for listing, and the continuous reform of pharmaceutical administration has accelerated the process of innovative drugs."
On December 17th, Junshi's triplizumab injection (trade name: Tuoyi) was conditionally approved by the State Drug Administration to be put on the market, becoming the first domestic PD-1 monoclonal antibody to treat patients with unresectable or metastatic melanoma after previous systemic treatment failure.
CITIC believes that the domestic PD-1/PD-L1 is expected to expand rapidly after listing, and the domestic market space is expected to exceed 40 billion yuan.The approved triplet monoclonal antibody injection was developed by Junshi Biology.Listed company Lepu Medical Strategy invests 198 million yuan to participate in Junshi Bio.
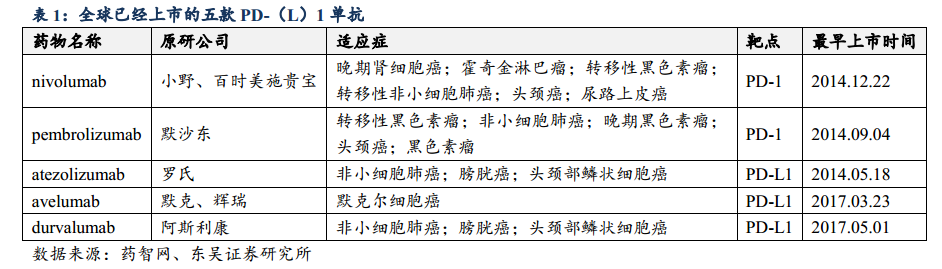
At present, five PD- (L) 1 McAbs have been listed around the world, Bristol-Myers Bristol-Myers Squibb Co is in the lead all the way, Merck & Co Inc is in hot pursuit, and pharmaceutical giants Roche, Merck and AstraZeneca PLC have entered the fighting situation one after another. The gap between Bristol-Myers Bristol-Myers Squibb Co and Merck & Co Inc is narrowing.
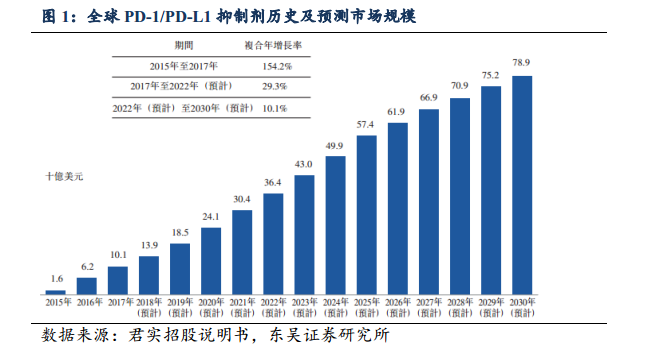
According to the Falls report, the global market for PD-1/PD-L1 inhibitors has grown rapidly since the commercialization of the first anti-PD-1 monoclonal antibody, coupled with the increase in indications and the popularity of drug combination therapy, the market will continue to grow in the next decade, with global market revenue growing at a compound rate of 154.2% (US $1.6 billion in 2015 to US $10.1 billion in 2017). The market is expected to reach US $78.9 billion in 2030.
After Opdivo and Keytruda, the first domestic PD-1 has been approved to go on the market, which is expected to usher in the peak of domestic innovative drugs.According to the 2018 quarterly report data disclosed by Shanghai Pharmaceutical, Shanghai Pharmaceutical obtained the domestic distributor rights of two PD-1 products Opdivo and Keytruda, as of September, the distribution income of the two products was 190 million yuan and 150 million yuan respectively, Soochow Securities judged that the domestic market of PD-1 products is vast, and the domestic market size of PD-1 and PD-L1 will grow to 37.4 billion yuan by 2022.In the first echelon of domestic McAbs, except for the conditional approval of this injection, the PD-1 McAbs of Cinda, Hengrui and Baiji have all been reported for production and are expected to be approved in the near future. The research and development level of domestic biopharmaceuticals is gradually catching up with multinational pharmaceutical companies.


Soochow Securities believes that with the good sales of imported PD-1 McAbs and the approval of domestic PD-1 McAbs, it is expected that other PD-1 McAb varieties will be approved to be listed in the near future, becoming a flexible source for the company's rapid growth in performance. It is recommended to pay attention to Hengrui Medicine (600276.SH) and so on.
