Source: China Fund Daily
Henan Real Biotechnology Co., Ltd. (hereinafter referred to as "Real Biology"), which was approved for the first domestic COVID-19 oral medicine just ten days ago, submitted its IPO application to Hong Kong Exchanges and Clearing on the evening of August 4.
The purpose of the IPO fund-raising includes the manufacture and commercialization of COVID-19 's core product Azvudine, as well as clinical development for the treatment of HIV infection, HFMD and several types of hematological tumors. In this prospectus, Real Biology did not disclose the number of shares to be issued and the amount of funds to be raised.
Financial data show that in 2020, 2021 and the first May of 2022, the company's income was 68000 yuan, 1.376 million yuan and 8.451 million yuan respectively, and the losses during the period were 151 million yuan, 197 million yuan and 218 million yuan respectively, of which R & D expenditure became the main source of loss.
Fund-raising to be used for COVID-19 's oral medicine commercialization
According to the prospectus$Real creature (temporary code) (810427.HK) $Founded in Pingdingshan City in 2012, it is an innovative drug research and development enterprise integrating independent research and development, production and sales, mainly engaged in the research and development of innovative drugs such as antiviral, anti-tumor, cardio-cerebrovascular and liver diseases.
According to the prospectus, the company's core product, Azvudine, is an innovative drug with broad-spectrum antiviral activity, which has been conditionally approved by the State Drug Administration for the treatment of HIV infection and COVID-19 in July 2021 and July 2022, respectively, and the first oral direct antiviral drug developed for a Chinese company approved by the State Drug Administration for the treatment of COVID-19. The company said it was fully prepared to start commercial sales of Azvudine.
According to the prospectus, in July 2022, the company entered into a strategic cooperation agreement with Shanghai Fosun Pharmaceutical Industries, a subsidiary of Shanghai Fosun Pharmaceutical, for joint development in mainland China and potential global regions and the exclusive commercialization of Shanghai Fosun Pharmaceutical Industries for the treatment and prevention of HIV infection and COVID-19.
In addition, the company has entered into strategic agreements with a number of leading pharmaceutical manufacturers in China, including Beijing Union Union, to produce and supply active pharmaceutical ingredients (API) and tablets of Azvudine for the company. The company also has its own production capacity, with an annual production capacity of about 1 billion tablets. The company believes that its combination of self-owned and contractual capacity will be able to produce sufficient quantities of Azvudine to meet market demand.
One of the fund-raising targets of this listing is the manufacture and commercialization of the company's core product Azvudine Therapeutic COVID-19, which is mainly used to finance the procurement of pharmaceutical raw materials for the commercial production of Azvudine and to expand the production capacity of the Pingdingshan production plant.
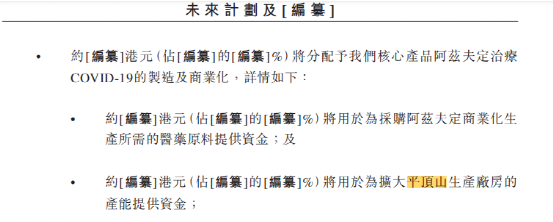
In addition, part of the funds raised will also be used for the clinical development of azivudine in the treatment of HIV infection, HFMD and several types of hematological tumors, complete oral long-acting compound tablets (azivudine ╱ CL-197) for the treatment of HIV infection in planned clinical trials, clinical development of preclinical or IND phase drug candidates, strengthening the research and development platform and expanding the product pipeline.
Three weeks of continuous "attack on cities and territories"
It is worth noting that from the publication of the results of the phase III clinical trial in the treatment of COVID-19, to the approval of the application for conditional registration, to the announcement of formal production, and then to the submission of the listing financing application, it was completed in only three weeks, which was unbelievably fast.
On July 15, Real Biology announced the results of the III phase clinical trial of azivudine in the treatment of COVID-19. The results showed that the rate of clinical symptom improvement on the 7th day after the first administration of azivudine was 40.43%, while that of the placebo group was 10.87%. There was a very significant difference in the median time of clinical symptom improvement between the azivudine group and the placebo group (P value).
On July 25th, the State Drug Administration conditionally approved the application for registration of COVID-19 for additional treatment of Azvudine tablets, and required the listing license holder to continue to carry out relevant research work, complete the conditional requirements within a time limit, and submit the follow-up research results in a timely manner.

Then, on August 2, the production ceremony of COVID-19 oral drug Azvudine tablets was held in Henan Real Biotechnology Co., Ltd., the urban-rural integration demonstration zone of Pingdingshan City, and announced that "Azvudine" had been put into production. Wang Chaoyang, founder of real biology, said that the approval of Azvudine anti-COVID-19 indications on the market is an important milestone in the development of real biology.
Two days later, on August 4, Real Biology submitted an application for listing to the Stock Exchange.
At present, Real Biology has signed strategic cooperation agreements with many domestic pharmaceutical companies, such as Xinhua Pharmaceutical, China Resources Shuanghe, Aoxiang Pharmaceutical, etc., but it has handed over the exclusive commercial rights to Shanghai Fosun Pharmaceutical.
Shanghai Fosun Pharmaceutical announced on July 25th that he had reached a strategic cooperation with Real Biology on promoting the joint development of the two sides and the exclusive commercialization of azivudine by Shanghai Fosun Pharmaceutical Industry. The above commercialization includes distribution, import, export, sales and promotion, and the areas of cooperation include novel coronavirus, AIDS treatment and prevention. The proposed cooperation areas are within China (excluding Hong Kong, Macao and Taiwan) and global regions (except Russia, Ukraine, Brazil and other South American countries and regions).
However, what is slightly strange is that in the face of such a great benefit, Shanghai Fosun Pharmaceutical ushered in a dive after opening sharply that day, but closed down more than 3%, leaving a long enema shade, and then fell nearly 15% in the next five trading days.
Lost 568 million yuan in two years and five months.
In terms of financial data, the company is in a period of continuous investment, with little revenue and continuing losses.
In 2020, 2021 and May before 2022, the company's "other income and income" was 68000 yuan, 1.376 million yuan and 8.451 million yuan, with losses of 151 million yuan, 197 million yuan and 218 million yuan respectively. The R & D expenditure is 106 million yuan, 64 million yuan and 114 million yuan respectively.

At the end of 2020 and the end of 2021, the company's net liabilities were 217 million yuan and 390 million yuan respectively. The company said that interest-bearing loans and other loans and accounting treatment of preferred shares resulted in convertible redeemable preferred shares under current liabilities. As of May 31, 2022, the company's net debt further rose to 564 million yuan, which was mainly due to 8.31 yuan of convertible redeemable preferred shares classified as non-current liabilities.
The above preferred shares mainly come from the company's two rounds of financing in 2020 and 2021, in which round A was given by Yifeng Capital alone, while round B continued to lead the investment of US $100 million.

Although the amount of financing and R & D investment is not small, the number of employees of the company does not seem to be large.
According to the prospectus, as of May 31, 2022, the company's internal R & D team was made up of 41 members. According to Sky Eye Survey data, 33 people will pay social security for the whole company in 2021.

Edit / new
