Edited by CICC Securities: "CAR-T cell therapy, hematological tumor shines brilliantly"
Tumor immunotherapy refers to the application of immunological principles and methods to improve the immunogenicity of tumor cells, stimulate and enhance the body's anti-tumor immune response, so as to inhibit tumor growth. Because of its small side effects and obvious curative effect, tumor immunotherapy is expected to become an innovation in the field of tumor therapy after surgery, chemotherapy, radiotherapy and targeted therapy. At present, tumor immunotherapy includes cytokines, tumor vaccine, adoptive cell therapy, oncolytic virus and immune checkpoint blocking.
1. CAR-T cell therapy, hematological tumor therapy is brilliant.
Cellular immunotherapy mainly refers to adoptive cell therapy (ACT), which can be divided into non-specific and specific, the former includes NK, CIK, etc., and the latter includes DC-CIK, TCR-T, CAR-T and so on. CAR-T therapy (Chimeric Antigen Receptor T-Cellimmunotherapy), the full name of chimeric antigen receptor T cell immunotherapy, is based on the principle that T cells obtained from patients are reinjected into the patient's body after activation, transfection, modification and amplification by genetic engineering, and the tumor is eliminated by stimulating the body's own immune system.
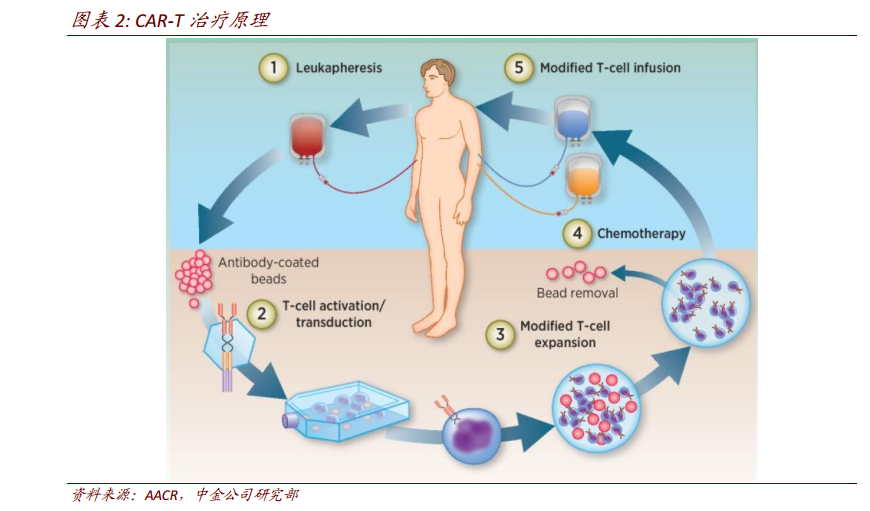
CD19-targeted CAR-T cells show amazing performance in the treatment of B-cell malignant tumors.. In the existing CAR-T therapy, the study of CAR-T cells targeting CD19 is the most and most mature. At the 2017 ASCO meetingThe application of CAR-T in r MM (multiple myeloma) also achieved remarkable results.94% (33x35) patients achieved VGPR (very good partial response, very good partial remission) within 2 months after CAR-T cell infusion.
2. CAR-T cell therapy is mainly divided into six steps.
1) T cells were isolated from peripheral blood of cancer patients.
2) T cells are activated by anti-CD3 and anti-CD28 antibodies.
3) transfer the CAR structure which can specifically recognize tumor cells into T cells by genetic engineering.
4) in vitro culture, a large number of CAR-T cells were expanded to the required dose for treatment, usually at the level of 1 billion to 10 billion.
5) chemotherapy and gonorrhea pretreatment.
6) reinfusion of CAR-T cells to observe the curative effect and closely monitor the adverse reactions.
3. Safety is the focus, and solid tumor treatment meets multiple challenges.
Lethality raises concerns. In March 2017, Juno, one of the leaders in CAR-T treatment, officially abandoned the phase Ⅱ clinical study of CAR-T product JCAR015 due to multiple deaths of patients with brain edema in clinical trials. In May 2017, Kite Pharmaceuticals CAR-T therapy KTE-C19 also reported a death of a patient with brain edema, which will undoubtedly have a negative impact on KTE-C19 that has applied for BLA. Therefore, in addition to the curative effect, the prevention and treatment of the toxic and side effects of CAR-T has also become the key factor to determine whether the technology can be put on the market.
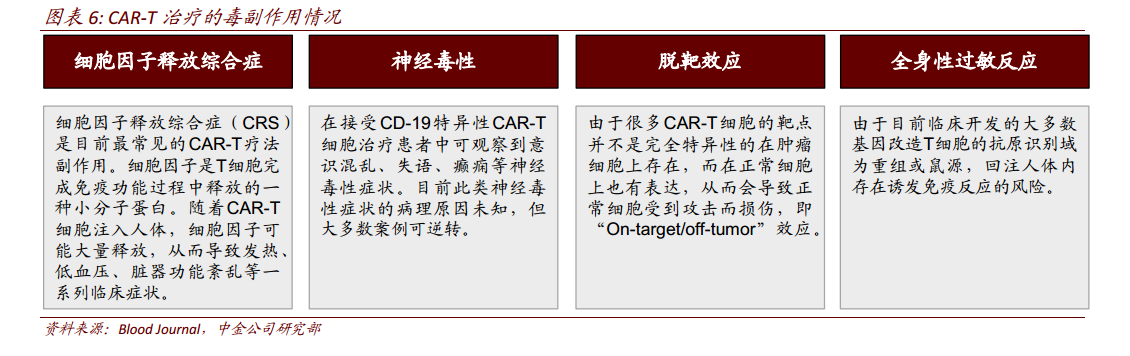
It is now known that the possible side effects of CAR-T treatmentIncluding cytokine release syndrome (CRS, Cytokine releasesyndrome), neurotoxicity (Neurological toxicity), miss effect (On-target/off-tumor recognition), systemic anaphylaxis (Anaphylaxis) and so on.

In addition, due to the existence of tumor microenvironment in solid tumors, CAR-T cells are often inactivated before reaching the target cells. In addition, due to the lack of specific TAA (tumor associated antigen) targets in solid tumors and the difficulty of chemotaxis of T cells in large tumors, CAR-T has many challenges in the treatment of solid tumors.
4. Novartis, Kite, Cellectis: the new big three take the lead in international CAR-T treatment
Since Professor Carl H June of the University of Pennsylvania invented CAR-T cell therapy, a number of international biotechnology companies have carried out further exploration of CAR-T. The first three giants to develop CAR-T cell therapy are Novartis, Kite Pharma and Juno Therapeutics. The initial research on CAR-T is focused on targeting CD19 and treating blood-related tumors.

On August 30, 2017, Novartis CAR-T Cell Therapy Kymriah (CTL019) was approved by FDA in the United States and became the first CAR-T product to be put on the market. In October 2017, Kite Pharma's Yescarta (axiaxicabtagene ciloleucel) was approved by FDA in the United States, becoming the second global CAR-T product to be approved after Novartis Kymriah (CTL019).
5. Many domestic enterprises are carrying out CAR-T research and development.
At present, many domestic enterprises have advanced their CAR-T R & D projects to the clinical stage, and the leading ones are Yucadi, Kingsley, Sibiman, Koji Biology, Boshengji, etc.; in addition to the traditional CAR-T cells for blood tumors, Keji Bio's treatment of hepatocellular carcinoma and Boshengji's development of CAR-NK technology are also quite unique. In addition, Shanghai Fosun Pharmaceutical and the international giant Kite Pharma established Fuxing Kate Biotechnology Co., Ltd., Wuxi Apptec and Juno to establish Pharmaceutical Mingjunuo (Shanghai) Co., Ltd., aiming to become a leading cell therapy company in China.

6. The international market space of hematological oncology is 9.3 billion US dollars, and the domestic space is 11.1 billion RMB.
According to the classification of lymphoma and leukemia, CICC Securities calculates in detail the market space of each subtype, and believes that the largest market for CAR-T treatment in the world is DLBCL, while there is a broader space for leukemia in China due to ethnic differences, especially AML is expected to become the largest market after a technological breakthrough (to solve the off-target effect). Considering the poor quality of life and high recurrence rate of patients with hematological tumors treated by first-line chemotherapy and stem cell transplantation, CICC Securities believes that with the continuous maturity of CAR-T technology, it is expected to gradually move towards the first-line treatment of hematological tumors in the future. In addition, with the continuous breakthrough of solid tumor technology, the future CAR-T market space is expected to reach tens of billions of dollars.
For more exciting content, please click: Fu Tu Research selected Topics
