Refined from Societe Generale Securities: "Anti-PD-1 McAb: new Star of Immunotherapy, domestic Development started in the first year"
The research on the treatment of cancer has always been a world-class research topic, and mankind has been making progress in the means of cancer treatment.At present, for the treatment of cancer, most of the common and routine treatments focus on surgery, chemotherapy, radiotherapy and targeted therapy which has achieved good results.Although these treatments have a certain effect in the treatment of cancer, but their effects are still far from satisfying the treatment of patients.
However, in recent years, a new type of anti-cancer therapy has created several miracles of "clinical cure" of advanced cancer one after another, and has become a "cardiotonic" in the fight against cancer. This new anti-cancer therapy is immunotherapy.Among them, the most eye-catching and clinical application is the immune checkpoint inhibitor PD-1/PD-L1 immunotherapy.
1. What is PD-1/PD-L1 immunotherapy?
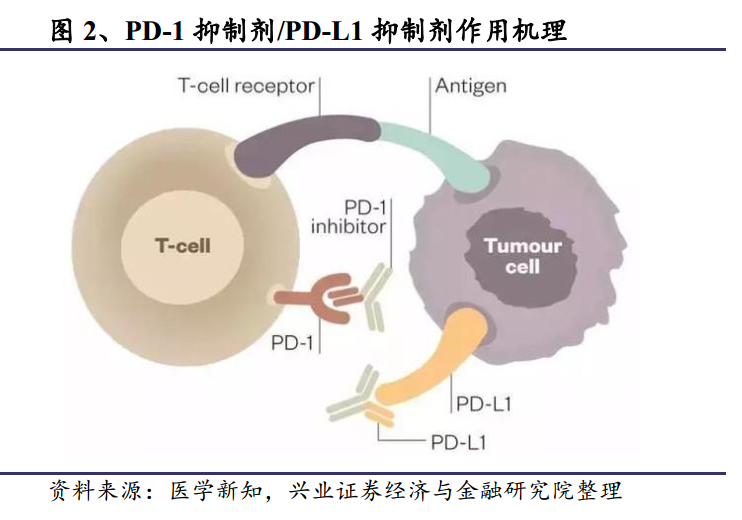
To put it simply, PD-1/PD-L1 immunotherapy is to block the PD-1/PD-L1 pathway by drugs and activate the body's own immune system to attack tumor cells.
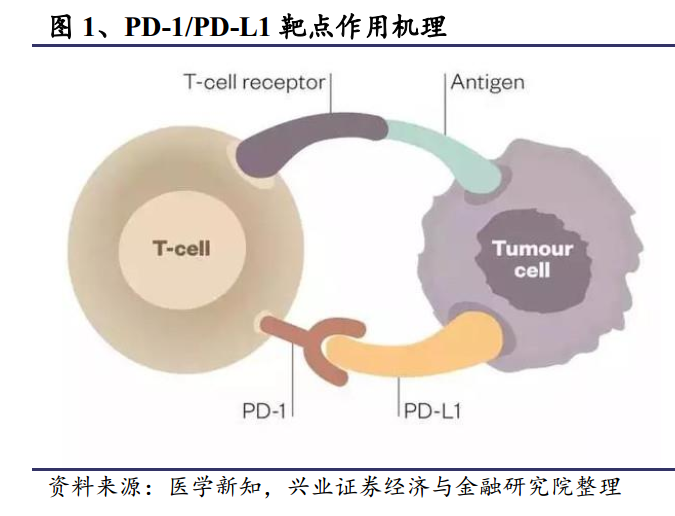
In fact, you might as well understand the PD-1/PD-L1 pathway here as a pair of "joint code words". The PD-1 receptor is the "Heavenly King cover Tiger" that activates T cells, and the PD-L1 receptor is the "Baota Zhenhe demon" of antigen-presenting cells. once the code word is docked successfully, the immune cells think that there is "no abnormality", so they will not launch an attack. This was originally a magical gift from evolution, but somehow, the cancer cell guy also secretly learned the code word, it can also identify the "pagoda town river demon" when it meets T cells, and successfully "whitewash peace" to deceive T cells. and finally avoid being attacked by immune cells.
In this case, PD-1/PD-L1 immunotherapy came out to help, using drugs to block the pathway, so that even if the tumor cells knew the code words, there was nothing they could do but to be attacked by immune cells as "alien molecules". Researchers have confirmed that most tumors express PD-L1 receptors, which means that PD-1/PD-L1 immunotherapy can theoretically contribute to the treatment of multiple tumors.
2. The listing of five McAbs has been approved to boost global sales
At present, there are five major PD-1/PD-L1 McAbs on the market, namely Opdivo, Keytruda, Tecentriq, Imfinzi and Bavencio. In 2017, the global market size of PD-1/PD-L1 McAbs is close to 10 billion US dollars.
The representative drugs of PD-1 are:Merck & Co Inc's pembrolizumab (trade name: Keytruda)It is the first PD-1 inhibitor on the market in the United States as a second-line therapy for advanced melanoma. At present, tumor immunotherapy is being studied in the fields of breast cancer, lymphoma, lung cancer, sarcoma, renal cell carcinoma, melanoma, colorectal cancer, bladder cancer, blood cancer, prostate cancer and bone marrow cancer.

Nibolumab, an inhibitor of PD-1 from Bristol-Myers Bristol-Myers Squibb Co (trade name: Opdivo)It was approved by FDA in 2011 and listed in Japan in July 2014 for the treatment of metastatic or unresectable melanoma.

The representative drugs of PD-L1 are:Tecentriq is the first PD-L1 inhibitor.

3. The approval of indications is the core driving force for the expansion of drug market.
Since being approved for listing in 2014, Opdivo and Keytruda have 10 and 7 indications respectively, occupying an absolute competitive advantage, with a compound growth rate of 129.19% and 159.42% respectively for three consecutive years. According to ResearchandMarkets's prediction,PD-1/PD-L1 sales will maintain a compound annual growth rate of 23.4% in the future, and are expected to reach $50 billion by 2025.


4. There are many kinds of PD-1/PD-L1 combined drugs and strong cooperation, and the potential market is expected to expand.
The combination of PD-1/PD-L1 monoclonal antibody and other tumor therapeutic agents can achieve a breakthrough in curative effect.From the current clinical results, the curative effect of combination therapy is significantly better than that of single drug in the treatment of non-small cell lung cancer, melanoma and renal cell carcinoma.And with the continuous use of combination drugs, the indications of clinical trials will be further expanded in the future.
5. 2018 is the first year for PD-1 McAb to be listed in China, and the market scale is expected to reach 45 billion yuan.
With the acceleration of priority approval of tumor targeting drugs in China, on June 15 and July 25, 2018, CFDA announced the approval of Bristol-Myers Bristol-Myers Squibb Co PD-1 McAb Opdivo and Merck & Co Inc PD-1 McAb Keytruda to be listed on the market, becoming the first two major PD-1 drugs on the market, which will vigorously promote other domestic enterprises to accelerate the research and development process of tumor immunotherapy drugs.Societe Generale Securities believes thatThe market space of new drugs on the market is expected to reach 45 billion yuan.

6. The competitive pattern of "44th" has taken shape, and the layout of R & D pipelines with high-quality domestic targets has been accelerated.
At present, the domestic market basically forms the competition pattern of "4 multinational pharmaceutical enterprises + 4 local innovative pharmaceutical enterprises". On the one hand, multinational pharmaceutical companies rely on the leading clinical progress and R & D technology, rapid layout in China; on the other hand, domestic local pharmaceutical companies compete to seize the PD-1/PD-L1 market, accelerating listing is just around the corner.Recommendation of Societe Generale SecuritiesPay attention to the companies with rapid progress in clinical trials, leading R & D capabilities and rich product portfolioFocus on four local innovative leading pharmaceutical companies: Hengrui Pharmaceutical, INNOVENT BIO, Junshi Bio, BeiGene, Ltd..

For more wonderful content, please mark: the past period of the rich way research election.
