Hepatitis B, hepatitis C and AIDS are the major diseases of all viral diseases, accounting for about 80% of the antiviral drug market by sales in 2017. Golly Pharmaceuticals focuses on the development and commercialization of best-in-class innovative drugs against HCV, HIV and HBV.
Refined from Societe Generale Securities: Golly Pharmaceuticals-Innovation-driven Integrated Anti-virus platform
Golly Pharmaceuticals: the company is an integrated antiviral platform focusing on the development and commercialization of best-in-class innovative drugs against HCV, HIV and HBV.
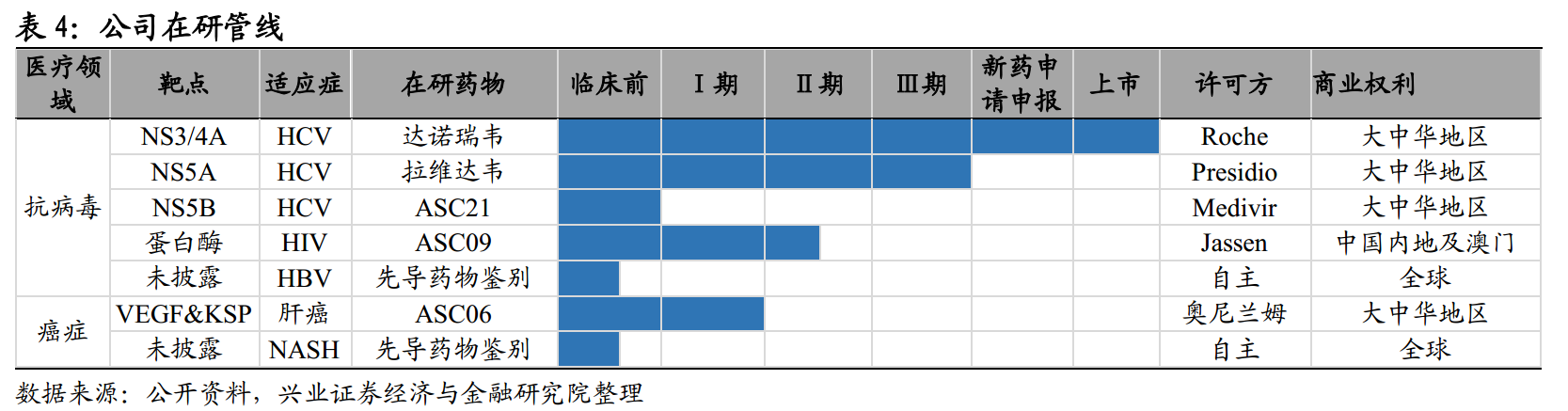
The company currently has five antiviral drug discovery and development programs, including two hepatitis C drugs in or near the commercial stage and one HIV drug that has completed Ⅱ a clinical trials. In addition, the company has a liver cancer drug under development that has completed phase Ⅰ and phase Ⅰ expanded clinical trials. The company's core products include Gonowei ®, Lavidave, ASC09 and ASC06.
Industry profile: rising penetration promotes continuous expansion of the antiviral market
In 2017, the market revenue of antiviral drugs in China was 26.2 billion yuan.The market of antiviral drugs mainly includes anti-hepatitis B, anti-hepatitis C and anti-AIDS drugs.The overall market for antivirals has grown steadily, with a compound annual growth rate of 10.9 per cent from 2013 to 2017.

Hepatitis B, hepatitis C and AIDS are the major diseases of all viral diseases, accounting for about 80% of the antiviral drug market by sales in 2017. One of the main reasons for the rapid expansion of the market is the increase in the number of patients receiving treatment. With the increase of incidence and treatment permeability, especially the increase of permeability, the number of patients receiving treatment for hepatitis C, liver cancer, AIDS and other diseases will continue to increase.

Competitive advantage: the company is developing pipelines, and the breakthrough excellent curative effect brings strong competitiveness.
1. The company's anti-hepatitis C drugs: Danorivir, lavedavir, and ASC21
Danorevir


Ravi Dawei


ASC21
ASC21 is a HCV nucleoside NS5B polymerase inhibitor waiting for clinical application for new drugs. It is a kind of nucleoside NS5B polymerase inhibitor which has been proved to be effective and pangenotypic with antiviral activity in vitro and has high gene resistance barrier.
Compared with Gilead developed for the treatment of chronic hepatitis C, the antiviral activity of ASC21 and the cure effect of HCV replicon encoded by drug resistance mutation were better than that of sofebvir.
2. The company's anti-AIDS drug: ASC09
ASC09 is a potential best protease inhibitor for the treatment of HIV-1 infection. ASC09 has unprecedented genetic resistance barrier, has completed phase Ⅰ and Ⅱ a clinical trials, and has shown effective antiviral activity. These clinical trials also showed the safety and good affordability of ASC09.

Dalunavir (DVR) is regarded as a high quality protease inhibitor among the approved protein inhibitors in the world. Virological studies have shown that ASC09 is a potential drug under development for 72% resistance to DRV clinical isolates, and Lopinavir is now the only protease inhibitor sold in China. Lopinavir has a low genetic barrier for resistance to protease inhibitors, so it is less effective for patients with protease inhibitor resistance. All these indicate that ASC09 has the potential to become a high quality protease inhibitor.
3. The company's anti-liver cancer drug: ASC06
ASC06 can inhibit the growth of two key genes of hepatocellular carcinoma cells-vascular endothelial growth factor ("VEGF") and spindle driver protein ("KSP"). ASC06 has completed clinical trials of phase Ⅰ and phase Ⅰ extension, and the results show that 50% of patients treated with ≥ 0.7mg/kg are in a nearly stable condition.

Risk Tips:
Financial outlook risk: the financial outlook for the next few years depends on the successful sales of Gonowei ®and the successful application and sale of lavedavir, and may face fierce competition in the antiviral drug market.
Risks related to the development, clinical trials and regulatory approval of drugs under study: regulatory approval of drugs under study may not be obtained, uncertainty of clinical trial results, etc.
Risks associated with commercialization of drugs under research: drugs under research may not be able to achieve the market recognition required for commercial success, may not be able to effectively establish and manage sales networks, and may encounter problems in production, etc.
Risks associated with the company's dependence on third parties
Risks related to the company's intellectual property rights
Business-related risks: may not be able to attract and retain senior management and research staff, it may be difficult to successfully manage our growth and expand our business, etc.
Risks related to doing business in China: changes in regulatory regulations, possible adverse effects of China's economic, political and social conditions and government policies, exchange rate fluctuations, etc.
For more wonderful content, please mark: the past period of the rich way research election.
