Editor's note: the writer is an analyst (Todd Campbell) from The Motley Fool.
$Dekang Medical (DXCM.US) $(DexCom), a medical equipment company, pioneered the development of ambulatory blood glucose monitors (CGM) for diabetics to more accurately monitor their blood sugar levels and control their diseases.
Dekang Medical was founded in 1999, and other giants also compete in the diabetic device market.$Medtronic PLC (MDT.US) $和$Abbott Laboratories (ABT.US) $. At present, there are more than 30 million people with diabetes in the United States alone, and 1.5 million cases of diabetes are diagnosed every year, so the number of people with diabetes in the United States is expected to exceed 50 million by 2030. With such a vast market, can Dekang Medical become a high-growth stock worth buying by investors?
What is diabetes?
In diabetics, the pancreas either does not secrete insulin properly or undersecretes it (type 1 diabetes), or the body itself becomes resistant to the insulin it produces (type 2 diabetes), unable to convert glucose intake into stored energy.
Type 1 diabetes is usually diagnosed in childhood or adolescence due to genetic or environmental factors. As a lifelong disease, type 1 diabetes requires long-term blood glucose monitoring and regular insulin doses to prevent a series of health problems caused by elevated blood sugar. Type 2 diabetes is usually diagnosed later than type 1 and is the most common type of diabetes, accounting for about 95% of patients with diabetes. Although type 2 patients do not need to monitor their blood sugar so intensively, it is also important to continuously analyze their blood sugar levels during treatment.
Without adequate blood glucose monitoring and improper use of insulin, people with diabetes face numerous health risks, including nerve damage that can lead to amputation, loss of vision, and life-threatening kidney and cardiovascular diseases. According to the World Health Organization (WHO), people with diabetes have a two to three times higher risk of heart attack and stroke than those without diabetes. Diabetes is the seventh leading cause of death. Overall, the average life expectancy of 50-year-old diabetics has been shortened by nearly 9 years.
How big is the market for diabetes?
According to the U.S. Centers for Disease Control (CDC)30.3 million Americans in the United States have diabetes.According to data from the World Health OrganizationWorldwide, the number of patients exceeds 422 million., up from 108 million in 1980.
It is expected that the number of people with diabetes will increase significantly in the next decade, so the total cost of treating diabetes will increase significantly. IAF's study concluded thatBy 2030, the number of people diagnosed or undiagnosed with diabetes in the United States will reach 54.9 million, while spending on diabetes will increase from $408 billion in 2015 to $622 billion by 2030.
Better blood glucose monitoring is needed for diabetes treatment.
The most important thing to maintain the health of patients with type 1 diabetes is to accurately monitor blood sugar and insulin dose to prevent blood sugar levels from being too high or too low.
Usually, people with type 1 and type 2 diabetes take blood samples from their fingertips to measure their blood sugar. Because patients with type 1 diabetes produce little or no insulin, they need to take blood samples four to 10 times a day, including regular blood sugar checks before eating, before and after exercise, before and after sleep, and even at night. Patients with type 2 diabetes do not need intensive monitoring, but they still regularly check their blood sugar levels, including before and after meals.
Although blood sugar is often checked with a "finger stick", studies have shown that most people with diabetes have blood sugar outside the health range for more than 70% of the day, leading to a higher risk of diabetes, which can be life-threatening.
Aware of the lack of blood sugar monitoring in many patients with diabetes, Dekang Medical's main task is to develop medical devices that dynamically monitor blood sugar levels so that patients can better determine when they should take insulin.
The Innovation History of Dekang Medical
Until 2003, Dekang Medical's research focused on the development of implantable sensors that could stay in the body for a long time. But in 2004, the company became increasingly concerned about getting approval for long-term implants, which required surgery and were more expensive, so the company turned to developing short-term temporary sensors that could be attached to the skin. In 2005, Dekang Medical raised $6.4 million on Nasdaq to fund short-term and long-term systematic clinical trials.
After the short-term sensor and CGM solution, the STS dynamic Blood glucose Monitoring (CGM) system, was approved by FDA in 2006, trials have shown that patients using the device spend more time in the normal healthy blood glucose range.
Short-term STS systems use tiny linear sensors that are inserted under the skin by patients and can be worn for three days. The data collected by these sensors are reported wirelessly to the STS receiver and displayed to the patient in the form of a chart. To help patients know whether their blood sugar is too high or too low, the STS system also includes a critical alarm function.
The company's second-generation product, the 7-day STS CGM system, was approved by FDA in 2007. The new product extends the time for diabetics to wear sensors and also allows patients to download blood sugar data to a computer for review.
These early systems provided revolutionary and innovative ideas, but they were not widely adopted until the results of the Adolescent Diabetes Research Foundation trial were published in 2008. In its trial of 322 people CGM significantly improved blood glucose control without hypoglycemia. The study is important because it reduces concerns that CGM use may lead to excessive insulin intake in patients.
Sales of Dekang Medical began to accelerate as there was growing evidence in support of the use of CGMs.In 2008, sales climbed to $8.1 million, up 76%, to $40 million in 2011.The rapid growth in sales has enabled Dekang Medical to invest more money in research and development, accelerating the development of its next generation of CGM products, the G-Series.
The first G-series CGM product is the G4 Platinum approved by FDA in 2012.The G4 is smaller than the previous STS system and improves its hypoglycemic accuracy by 30 per cent.Accuracy is important because the CGM cannot measure glucose levels directly from the blood, but rather the CGM sensor is inserted into the interstitial tissue under the skin to infer the actual blood sugar level immediately from the sensor readings. A lack of accuracy can lead to patients taking too much insulin, resulting in low blood sugar. The G4 also extends the transmission distance limit between the sensor and the receiver and uses a more user-friendly color LCD display.
In 2015, Dekang Medical followed the G4 and launched the G5. The G5 allows patients to view real-time data on Dekang Healthcare receivers or any compatible device, including smartphones, shares real-time data with caregivers, such as parents, and provides access to data cloud storage for review. The important thing is thatThe G5 is the first product in the Dekang Medical CGM that reduces the need for a hand pointer prick test before taking insulin.. Although finger acupuncture is still needed every 12 hours to calibrate system readings this progress has significantly reduced the burden of finger acupuncture on patients.
Continued technological advances in products such as G4 and G5, as well as increased general acceptance of CGM, have been very beneficial to the company's revenue growth. Since 2008Dekang Medical's annual sales increased from less than US $10 million to US $718.5 million in 2017, with a compound annual growth rate of nearly 57%.。
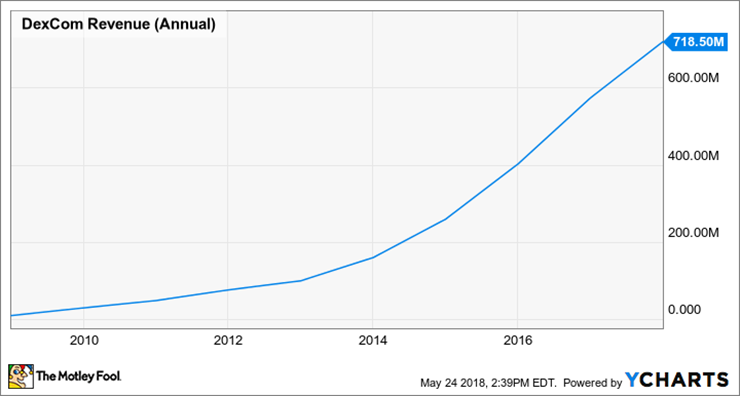
Dekang Medical Annual income / Source: YCHARTS
Competition intensifies: Medtronic PLC and Abbott Laboratories compete together
In the market of dynamic blood glucose monitoring equipment, Dekang Medical is not the only one. Medtronic PLC and Abbott Laboratories have also recently launched eye-catching new diabetes devices, which will pose a challenge to Dekang Medical.
Medtronic PLC launched the MiniMed 670G in 2017 after obtaining early approval in 2016. The MiniMed 670G is the first closed-loop monitoring and insulin dose system in the world to win the regulatory green light.It combines Medtronic PLC's Guardian dynamic blood glucose monitoring system and Medtronic PLC's insulin pump into one system, which automatically measures blood sugar levels every five minutes and then automatically gives insulin doses if necessary.
MiniMed 670G marks a major breakthrough in the treatment of people with diabetes who need extensive monitoring. In the fourth quarter of Medtronic PLC's fiscal year 2018As a result of the system's contribution, diabetes revenue rose 26% to $645 million.
Abbott Laboratories's latest dynamic blood glucose monitoring system Freestyle Libre has also ushered in a growing demand after it was approved. When Freestyle Libre was approved by the US Food and Drug Administration in 2017, it was the first CGM that did not need to be calibrated every day with a hand thorn. The sensor of the device has a 12-hour warm-up period during which the data are not recorded, so the support of the finger acupuncture test is required during this period.
The Freestyle Libre has been commercially successful because its launch price is lower than that of the Dekang Medical G5 and does not need to be calibrated with a hand thorn.As of March 2018, 650000 patients are using Freestyle Libre, with 50, 000 new patients per month. Abbott Laboratories did not specify how much Freestyle Libre contributed to its revenue, but said it contributed 30 per cent to diabetes revenue in the first quarter of 2018. Abbott Laboratories lists diabetes sales in the "other" category. Abbott Laboratories's total sales in the first quarter of 2018 were $7.4 billion, of which "other" income accounted for $430 million.
The Future of Dekang Medical
As you can see in the chart below, Abbott Laboratories's approval of Freestyle Libre in 2017 had an impact on Dekang Medical's share price. However, after FDA approved its latest CGM G6 in March 2018, Dekang Medical shares rose sharply, proving that the previous sell-off was temporary.

Source: Futu Securities
The new product G6 has more functions than Abbott Laboratories's Freestyle Libre. Like Freestyle Libre, Dekang Medical's G6 does not require finger acupuncture calibration, but unlike Abbott Laboratories's CGMThe G6 sensor reduces the warm-up period to two hours.. G6 is between its sensor and receiver.Automatically transmits data, and the receiver can also be a smartphone or similar device. By contrast, Abbott Laboratories's receiver must be kept within 1.5 inches of its sensor to transmit data, and cannot use a smartphone or tablet as a receiver. In addition, the G6 is inAn alarm will be given to patients when there is a dangerous critical blood glucose level.And Freestyle Libre won't.
The G6 system uses a smaller sensor than Dekang's previous G5, which can be applied to the skin through a new, easy-to-use single-touch sprinkler. In addition, the G6 sensor can be worn for 10 days, while the G5 sensor can only be used for 7 days.
The characteristics of G6 can help Dekang Medical replace Abbott Laboratories Freestyle Libre.But the most exciting thing is that the G6 is the first independent CGM approved by FDA, which means it can be paired with products from other insulin pump manufacturers.
At present, Medtronic PLC claims to be the only company with closed-loop monitoring and insulin delivery system (the above-mentioned MiniMed 670G), but this closed-loop is only used in pairing with Medtronic PLC's own insulin pump. While other insulin pump manufacturers such as$Insulet Corp. (PODD.US) $和$Tandem Diabetes Care, Inc. (TNDM.US) $Is developing its own closed-loop system and intends to design it for Dekang Medical's CGM.
Insulet is working on Omnipod insulin pumps paired with the G5, and its closed-loop system could be available after the end of 2019, according to management.
At the same time, the process of Tandem is one step faster. Its t:slim X2 insulin pump is the only pump approved for use with the Dekang G5 and does not require a finger prick test before adding insulin, while Tandem said the closed-loop system approved by FDA for the G5 could be available in the summer of 2018. The G6 system will be available by the end of 2018 or early 2019.
However, the potential associated with the Dekang Medical G6 is not limited to these two solutions. Dekang Medical is considering using it in conjunction with other solutions, such as smart insulin pens or applications.
The innovation of Dekang Medical will not stop. The company is working with Alphabet$Alphabet Inc-CL C (GOOG.US) $Verily, a healthcare subsidiary, works closely to provide an one-off CGM solution that could be on the market by 2020.
Dekang Medical ServiceIs it worth buying?
Dekang Medical is at the forefront of CGM technology, andThe cooperation strategy with Insulin delivery Systems can help it gradually gain more market share.。
However, the company is not yet profitable and there is no guarantee that its sales will reach the level necessary to generate benefits. Even so,The sheer size of the diabetes medical device market and the penetration of CGM in the market suggest that Dekang Healthcare has the potential to generate sufficient revenue to support shareholder profits.
There is no denying that Dekang Medical will face heavyweight competitors such as Medtronic PLC and Abbott Laboratories, but unlike these companies, Dekang Medical focuses on the market for medical devices for diabetes. If CGM becomes the standard of treatment for diabetics along with insulin delivery, its investors can reap substantial benefits. For this reason, the author Dekang Medical stock is worth owning.
(this article is produced by Futu Information compilation team, compiled / Ni Jingwen, proofread / Yang Weiyi)
